Abstract
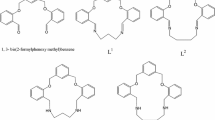
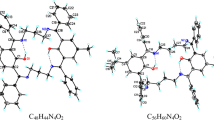
Three new macrocyclic Schiff bases containing an amine or amide structural fragment along with imine groups were synthesized by condensation of 2,6-bis(2-aminophenyliminomethyl)pyridine (1) and N, N’-bis(2-aminophenyl)pyridine-2,6-dicarboxamide (2) with 2,5-diformylpyrrole (3) and 2,2-bis(5-formylpyrrol-2-yl)propane (4). The reaction of compound 1 with 3 proceeds abnormally and is accompanied by redox disproportionation of compound 1 in the first step. The structure of the macrocyclic product of this reaction was established by X-ray diffraction analysis. Spectrophotometric titration showed that hybrid macrocycle 10, which was prepared by condensation of compound 2 with 4, possesses the properties of an anion receptor and selectively binds hydrosulfate and dihydrophosphate anions in the presence of bromide and nitrate anions. The structures of 10 and its adduct with the hydrosulfate anion were calculated by density functional theory.
Similar content being viewed by others
References
J.-M. Lehn, Supramolecular Chemistry. Concept and Perspectives, VCH, Weinheim, 1995.
A. Bianchi, K. Bowman-James, and E. Garcia-Espaca, Supramolecular Chemistry of Anions, Wiley-VCH, New York, 1997.
P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed. Engl., 2001, 40, 486.
R. Martinez-Macez and F. Sancenon, Chem. Rev., 2003, 103, 4419.
P. A. Gale, J. L. Sessler, V. Kral, and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140.
J. L. Sessler, P. J. Anzenbacher, Jr., J. A. Shriver, K. Jursikova, V. M. Lynch, and M. Marquez, J. Am. Chem. Soc., 2000, 122, 12061.
M. Van Kuijck, H. Miyaji, and J. L. Sessler, Supramolecular Chemistry, 2002, 13, 661.
W. Sato, H. Miyaji, and J. L. Sessler, Tetrahedron Lett., 2000, 41, 6731.
A. Andrievsky, F. Ahuis, J. L. Sessler, F. Voegtle, D. Gudat, and M. Moini, J. Am. Chem. Soc., 1998, 120, 9712.
J. L. Sessler, W.-S. Cho, S. P. Dudek, L. Hicks, V. M. Lynch, and M. T. Huggins, J. Porphyrins Phthalocyanines, 2003, 7, 97.
C.-H. Lee, H. K. Na, D.-W. Yoon, D.-H. Won, W.-S. Cho, V. M. Lynch, S. V. Shevchuk, and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 7301.
Yu. A. Ustynyuk, N. E. Borisova, V. M. Nosova, M. D. Reshetova, S. S. Talismanov, S. E. Nefedov, G. G. Aleksandrov, I. L. Eremenko, and I. I. Moiseev, Izv. Akad. Nauk, Ser. Khim., 2002, 454 [Russ. Chem. Bull., Int. Ed., 2002, 51, 488].
N. E. Borisova, Yu. A. Ustynyuk, M. D. Reshetova, G. G. Aleksandrov, I. L. Eremenko, and I. I. Moiseev, Mendeleev Commun., 2003, 2002.
N. E. Borisova, M. D. Reshetova, and Yu. A. Ustynyuk, Izv. Akad. Nauk, Ser. Khim., 2004, 174 [Russ. Chem. Bull., Int. Ed., 2004, 53, 181].
E. A. Katayev, M. D. Reshetova, and Yu. A. Ustynyuk, Izv. Akad. Nauk, Ser. Khim., 2004, 322 [Russ. Chem. Bull., Int. Ed., 2004, 53, 335].
N. E. Borisova, Yu. A. Ustynyuk, M. D. Reshetova, G. G. Aleksandrov, I. L. Eremenko, and I. I. Moiseev, Izv. Akad. Nauk, Ser. Khim., 2004, 326 [Russ. Chem. Bull., 2004, Int. Ed., 53, 340].
J. L. Sessler, E. Katayev, G. D. Pantos, and Yu. A. Ustynyuk, Chem. Commun., 2004, 1276.
C. Picard, N. Arnaud, and P. Tisnes, Synthesis, 2001, 1471.
K. Kavallieratos, C. M. Bertao, and R. H. Crabtree, J. Org. Chem., 1999, 64, 1675.
K. Niikura, A. P. Bisson, and E. V. Anslyn, J. Chem. Soc., Perkin Trans. 2, 1999, 1111.
J. B. Love, A. J. Blake, C. Wilson, S. D. Reid, A. Novak, and P. B. Hitchcock, Chem. Commun., 2003, 1682.
K. Brychcy, K. Draeger, K.-J. Jens, M. Tilset, and U. Behrens, Chem. Ber., 1994, 127, 465.
F. Benetollo, G. Bombieri, K. K. Fonda, A. Polo, J. R. Quagliano, and L. M. Vallarino, Inorg. Chem., 1991, 30, 1345.
G. J. Kirkovits, R. S. Zimmerman, M. T. Huggins, V. M. Lynch, and J. L. Sessler, Eur. J. Org. Chem., 2002, 3768.
J. L. Sessler, G. D. Pantos, E. Katayev, and V. M. Lynch, Org. Lett., 2003, 5, 4141.
K. A. Connors, Binding Constants; John Wiley & Sons, New York, 1987, (a) p. 148; (b) p. 24.
P. D. Beer, D. Hesek, and K. C. Nam, Organometallics, 1999, 18, 3933.
K. Choi and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 2456.
S. Kubik, R. Kirchner, D. Nolting, and J. Seidel, J. Am. Chem. Soc., 2002, 124, 12752.
H. Ihm, S. Yun, H. G. Kim, J. K. Kim, and K. S. Kim, Org. Lett., 2002, 4, 2897.
R. Herges, A. Dikmans, U. Jana, F. Kohler, P. G. Jones, I. Dix, T. Fricke, and B. Konig, Eur. J. Org. Chem., 2002, 3004.
D. H. Lee, H. Y. Lee, and J.-I. Hong, Tetrahedron Lett., 2002, 43, 7273.
S. O. Kang, J. M. Oh, Y. S. Yang, J. C. Chun, S. Jeon, and K. C. Nam, Bull. Korean Chem. Soc., 2002, 23, 145.
S. J. Coles, G. Denuault, P. A. Gale, P. N. Horton, M. B. Hursthouse, M. E. Light, and C. N. Warriner, Polyhedron, 2003, 22, 699.
S. O. Kang, J. M. Llinares, D. Powell, D. Vander Velde, and K. Bowman-James, J. Am. Chem. Soc., 2003, 125, 10152.
S. L. Tobey and E. V. Anslyn, J. Am. Chem. Soc., 2003, 125, 14807.
Y. S. Yang, S. W. Ko, I. H. Song, B. J. Ryu, and K. C. Nam, Bull. Korean Chem. Soc., 2003, 24, 681.
J. D. Vienna, M. J. Schweiger, D. E. Smith, H. D. Smith, J. V. Crum, D. K. Peeler, I. A. Reamer, C. A. Musick, and R. D. Tillotson, Report PNNL-12234, Pacific Northwest National Laboratory, Richland, Washington, July 1999.
C. I. Crawford, D. M. Ferrara, R. F. Schumacher, and N. E. Bibler, Report WSRC-MS-2002-00449, Westinghouse Savannah River Company, Aiken, South Carolina, Apr. 2002.
D. E. Kurath, J. R. Bontha, D. L. Blanchard, S. K. Fiskum, and B. M. Rapko, Report PNWD-3053, BNFL-RPT-036, Rev. 0, Pacific Northwest National Laboratory, Richland, Washington, USA, August 2000.
B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, J. M. Llinares, A. Hossain, S. O. Kang, and K. Bowman-James, Supramolecular Chemistry of Environmentally Relevant Anions, ACS Symp. Ser., American Chemical Society, Washington, D. C., 2005–2006.
M. Freemantle, Chem. Eng. News, 2004, 82, No.23, 8.
R. Miller and K. Olsson, Acta Chem. Scand. Ser. B, 1981, 35, 303.
Z. Otwinowski and W. Minor, in Methods in Enzymology, 276: Macromolecular Crystallography, Part A, Eds C. W. Carter, Jr., and R. M. Sweets, Academic Press, New York, 1997, p. 307.
A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and R. J. Spagna, J. Appl. Cryst., 1999, 32, 115.
G. M. Sheldrick, SHELXL97. Program for the Refinement of Crystal Structures, University of Gottingen, Gottingen Germany, 1994.
Author information
Authors and Affiliations
Additional information
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 161–168, January, 2005.
Rights and permissions
About this article
Cite this article
Katayev, E.A., Pantos, G.D., Lynch, V.M. et al. New polydentate macrocyclic ligands of hybrid amine-imine and amide-imine types as artificial anion receptors. Synthesis and study of anion binding. Russ Chem Bull 54, 165–172 (2005). https://doi.org/10.1007/s11172-005-0233-4
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0233-4