Abstract
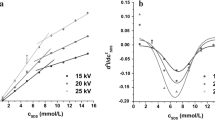
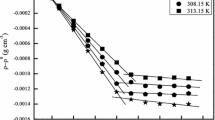
The critical micelle concentration (CMC) can be obtained by measuring the distinct physical properties of surfactant molecules in the monomeric and micellar states. In this study, two linear increments of relative viscosity with distinct slopes were obtained when increasing surfactant concentrations from dilute solution to above the CMC, which was then determined by the intersection of the two linear extrapolations. Using a capillary electrophoresis (CE) instrument and Poiseuille’s law, the viscosities of surfactants at a series of concentrations covering the monomeric and micellar regions could be obtained by measuring the hydrodynamic flow rates of the corresponding surfactant solutions. We applied this method to determine the CMC values of various types of surfactants including anionic, cationic, zwitterionic, and nonionic surfactants. The resulting CMC values were all in good agreement with those reported in literature. Using this method, the multiple-stage micellization process of a short-chain surfactant was revealed. We have also demonstrated that the CE-based viscometer was applicable to the study of CMC variation caused by organic or electrolyte additives.




Similar content being viewed by others
References
T.F. Tadros, Applied Surfactants: Principles and Applications, 1st edn. (Wiley, Weinheim, 2005), pp. 1–16
E. Calvo, R. Bravo, A. Amigo, J. Gracia-Fadrique, Fluid Phase Equilib. 282, 14 (2009)
E. Fuguet, C. Rafols, M. Roses, E. Bosch, Anal. Chim. Acta 548, 95 (2005)
U. Anand, C. Jash, S. Mukherjee, J. Colloid Interface Sci. 364, 400 (2011)
R.E. Stark, P.D. Leff, S.G. Milheim, A. Kropf, J. Phys. Chem. 88, 6063 (1984)
C.-E. Lin, J. Chromatogr. A 1037, 467 (2004)
M.S. Bello, R. Rezzonico, P.G. Righetti, J. Chromatogr. A 659, 199 (1994)
S. Priyanto, G.A. Mansoori, A. Suwono, Chem. Eng. Sci. 56, 6933 (2001)
F.E. Stanley, A.M. Warner, E. Schneiderman, A.M. Stalcup, J. Chromatogr. A 1216, 8431 (2009)
E. Cordova, J. Gao, G.M. Whitesides, Anal. Chem. 69, 1370 (1997)
A. Imhof, A. van Blaaderen, G. Maret, J. Mellema, J.K.G. Dhont, J. Chem. Phys. 100, 2170 (1994)
M.A. Lauffer, J. Am. Chem. Soc. 66, 1188 (1944)
A. Evilevitch, V. Lobaskin, U. Olsson, P. Linse, P. Schurtenberger, Langmuir 17, 1043 (2001)
H. Chen, Y. Ding, Y. He, C. Tan, Chem. Phys. Lett. 444, 333 (2007)
M.L. Corrin, W.D. Harkins, J. Am. Chem. Soc. 69, 683 (1947)
L. Xu, E. Yokoyama, M. Satoh, Langmuir 21, 7153 (2005)
M.J. Schick, J. Phys. Chem. 68, 3585 (1964)
M. Abu-Hamdiyyah, K.J. Kumari, Phys. Chem. 94, 6445 (1990)
J.C. Jacquier, P.L. Desbene, J. Chromatogr. A 718, 167 (1995)
G. Mangiapia, D. Berti, P. Baglioni, J. Teixeira, L. Paduano, J. Phys. Chem. B 108, 9772 (2004)
L.V. Dearden, E.M. Woolley, J. Chem. Thermodyn. 28, 1283 (1996)
B. Lindman, N. Kamenka, M.-C. Puyal, B. Brun, B. Jonsson, J. Phys. Chem. 88, 53 (1984)
A. Gonzalez-Perez, J.M. Ruso, G. Prieto, F. Sarmiento, Colloid Polym. Sci. 282, 1133 (2004)
M.F. Emerson, A. Holtzer, J. Phys. Chem. 69, 3718 (1965)
R. Sabate, M. Gallardo, J. Estelrich, Electrophoresis 21, 481 (2000)
A.K. Singh, M. Darshi, S. Kanvah, J. Phys. Chem. A 104, 464 (2000)
A. Zdziennicka, K. Szymczyk, J. Krawczyk, B. Janczuk, Fluid Phase Equilib. 322–323, 126 (2012)
A. Chattopadhyay, K.G. Harikumar, FEBS Lett. 391, 199 (1996)
Q. Guan, S.D. Noblitt, C.S. Henry, Electrophoresis 33, 379 (2012)
J.C. Gertsch, S.D. Noblitt, D.M. Cropek, C.S. Henry, Anal. Chem. 82, 3426 (2010)
B. Rozycka-Roszak, P. Misiak, B. Jurczak, K.A. Wilk, J. Phys. Chem. B 112, 16546 (2008)
A. Vishnyakov, M.-T. Lee, A.V. Neimark, J. Phys. Chem. Lett. 4, 797 (2013)
C. Wu, T. Liu, B. Chu, Macromolecules 30, 4574 (1997)
P. Alexandridus, J.F. Holzwarth, T.A. Hatton, Macromolecules 27, 2414 (1994)
T. Zemb, M. Drifford, M. Hayoun, A. Jehanno, J. Phys. Chem. 87, 4524 (1983)
E. Ruckenstein, R. Nagarajan, J. Phys. Chem. 85, 3010 (1981)
M.A. Desando, L.W. Reeves, Can. J. Chem. 64, 1817 (1986)
G.K. Batchelor, J.T. Green, J. Fluid Mech. 56, 401 (1972)
P. Ekwall, P. Holmberg, Acta Chem. Scand. 19, 455 (1965)
L. Moreira, A. Firoozabadi, Langmuir 26, 15177 (2010)
Acknowledgments
We thank the National Science Council of Taiwan and Tamkang University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, C., Li, N.J., Chen, K.C. et al. Determination of critical micelle concentrations of ionic and nonionic surfactants based on relative viscosity measurements by capillary electrophoresis. Res Chem Intermed 40, 2371–2379 (2014). https://doi.org/10.1007/s11164-014-1614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1614-9