Abstract
Objective
Previous work in pediatric oncology has found that clinicians and parents tend to under-report the frequency and severity of treatment-related symptoms compared to child self-report. As such, there is a need to identify high-quality self-report instruments to be used in pediatric oncology research studies. This study’s objective was to conduct a systematic literature review of existing English language instruments used to measure self-reported symptoms in children and adolescents undergoing cancer treatment.
Methods
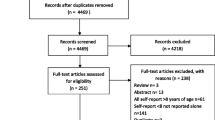
A comprehensive literature search was conducted in MEDLINE/PubMed, EMBASE, CINAHL, and PsycINFO to identify relevant articles published through November 10, 2016. Using pre-specified inclusion/exclusion criteria, six trained reviewers carefully screened abstracts and full-text articles for eligibility.
Results
There were 7738 non-duplicate articles identified in the literature search. Forty articles met our eligibility criteria, and within these articles, there were 38 self-report English symptom instruments. Most studies evaluated only cross-sectional psychometric properties, such as reliability or validity. Ten studies assessed an instrument’s responsiveness or ability to detect changes in symptoms over time. Eight instruments met our criteria for use in future longitudinal pediatric oncology studies.
Conclusions
This systematic review aids pediatric oncology researchers in identifying and selecting appropriate symptom measures with strong psychometric evidence for their studies. Enhancing the child’s voice in pediatric oncology research studies allows us to better understand the impact of cancer and its treatment on the lives of children.

Similar content being viewed by others
References
American Cancer Society. (2016). Cancer facts & figures 2016 (p. 11). Atlanta: American Cancer Society.
CureSearch Childhood Cancer Statistics http://curesearch.org/Childhood-Cancer-Statistics.
Sateren, W. B., Trimble, E. L., Abrams, J., Brawley, O., Breen, N., Ford, L., et al. (2002). How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of Clinical Oncology, 20(8), 2109–2117.
Bleyer, A., Budd, T., & Montello, M. (2006). Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer, 107(7 Suppl), 1645–1655. doi:10.1002/cncr.22102.
Collins, J. J., Byrnes, M. E., Dunkel, I. J., Lapin, J., Nadel, T., Thaler, H. T., et al. (2000). The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management, 19(5), 363–377.
Docherty, S. L. (2003). Symptom experiences of children and adolescents with cancer. Annual Review of Nursing Research, 21, 123–149.
Eiser, C., Hill, J. J., & Vance, Y. H. (2000). Examining the psychological consequences of surviving childhood cancer: Systematic review as a research method in pediatric psychology. Journal of Pediatric Psychology, 25(6), 449–460.
Linder, L. A. (2005). Measuring physical symptoms in children and adolescents with cancer. Cancer Nursing, 28(1), 16–26.
Pickard, A. S., Topfer, L. A., & Feeny, D. H. (2004). A structured review of studies on health-related quality of life and economic evaluation in pediatric acute lymphoblastic leukemia. Journal of the National Cancer Institute Monographs, 2004(33), 102–125. doi:10.1093/jncimonographs/lgh002.
Ruland, C. M., Hamilton, G. A., & Schjodt-Osmo, B. (2009). The complexity of symptoms and problems experienced in children with cancer: A review of the literature. Journal of Pain and Symptom Management, 37(3), 403–418. doi:10.1016/j.jpainsymman.2008.03.009.
Vance, Y. H., & Eiser, C. (2002). The school experience of the child with cancer. Child: Care, Health and Development, 28(1), 5–19.
Waters, E., Stewart-Brown, S., & Fitzpatrick, R. (2003). Agreement between adolescent self-report and parent reports of health and well-being: Results of an epidemiological study. Child: Care, Health and Development, 29(6), 501–509.
U.S. Department of Health and Human Services, F. A. D. A. (2009). Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. In F. A. D. A. U.S. Department of Health and Human Services (Ed.).
Hinds, P. S. (2016). In B. B. Reeve (Ed.), (Email ed.).
Hockenberry, M. J., Hinds, P. S., Barrera, P., Bryant, R., Adams-McNeill, J., Hooke, C., et al. (2003). Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management, 25(4), 319–328.
Le Gales, C., Costet, N., Gentet, J. C., Kalifa, C., Frappaz, D., Edan, C., et al. (1999). Cross-cultural adaptation of a health status classification system in children with cancer. First results of the French adaptation of the Health Utilities Index Marks 2 and 3. International Journal of Cancer Supplement, 12, 112–118.
Glaser, A. W., Davies, K., Walker, D., & Brazier, D. (1997). Influence of proxy respondents and mode of administration on health status assessment following central nervous system tumours in childhood. Quality of Life Research, 6(1), 43–53.
Parsons, S. K., Barlow, S. E., Levy, S. L., Supran, S. E., & Kaplan, S. H. (1999). Health-related quality of life in pediatric bone marrow transplant survivors: According to whom? International Journal of Cancer Supplement, 12, 46–51.
Collins, J. J., Devine, T. D., Dick, G. S., Johnson, E. A., Kilham, H. A., Pinkerton, C. R., et al. (2002). The measurement of symptoms in young children with cancer: The validation of the Memorial Symptom Assessment Scale in children aged 7-12. Journal of Pain and Symptom Management, 23(1), 10–16.
Yeh, C. H., Chang, C. W., & Chang, P. C. (2005). Evaluating quality of life in children with cancer using children’s self-reports and parent-proxy reports. Nursing Research, 54(5), 354–362.
Upton, P., Lawford, J., & Eiser, C. (2008). Parent-child agreement across child health-related quality of life instruments: A review of the literature. Quality of Life Research, 17(6), 895–913. doi:10.1007/s11136-008-9350-5.
Russell, K. M., Hudson, M., Long, A., & Phipps, S. (2006). Assessment of health-related quality of life in children with cancer: Consistency and agreement between parent and child reports. Cancer, 106(10), 2267–2274. doi:10.1002/cncr.21871.
Levi, R. B., & Drotar, D. (1999). Health-related quality of life in childhood cancer: Discrepancy in parent-child reports. International Journal of Cancer Supplement, 12, 58–64.
Sawyer, M., Antoniou, G., Toogood, I., & Rice, M. (1999). A comparison of parent and adolescent reports describing the health-related quality of life of adolescents treated for cancer. International Journal of Cancer Supplement, 12, 39–45.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. doi:10.1371/journal.pmed1000097.
Reeve, B. B., Withycombe, J. S., Baker, J. N., Hooke, M. C., Lyons, J. C., Mowbray, C., et al. (2013). The first step to integrating the child’s voice in adverse event reporting in oncology trials: A content validation study among pediatric oncology clinicians. Pediatric Blood & Cancer, 60(7), 1231–1236. doi:10.1002/pbc.24463.
Mokkink, L. B., Terwee, C. B., Knol, D. L., Stratford, P. W., Alonso, J., Patrick, D. L., et al. (2010). The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Medical Research Methodology, 10, 22. doi:10.1186/1471-2288-10-22.
Matza, L. S., Patrick, D. L., Riley, A. W., Alexander, J. J., Rajmil, L., Pleil, A. M., et al. (2013). Pediatric patient-reported outcome instruments for research to support medical product labeling: Report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health, 16(4), 461–479. doi:10.1016/j.jval.2013.04.004.
Aaronson, N., Alonso, J., Burnam, A., Lohr, K. N., Patrick, D. L., Perrin, E., et al. (2002). Assessing health status and quality-of-life instruments: Attributes and review criteria. Quality of Life Research, 11(3), 193–205.
Reeve, B. B., McFatrich, M., Pinheiro, L. C., Weaver, M. S., Sung, L., Withycombe, J. S., et al. (2017). Eliciting the child’s voice in adverse event reporting in oncology trials: Cognitive interview findings from the pediatric patient-reported outcomes version of the common terminology criteria for adverse events initiative. Pediatric Blood & Cancer. doi:10.1002/pbc.26261.
Hinds, P. S., Nuss, S. L., Ruccione, K. S., Withycombe, J. S., Jacobs, S., DeLuca, H., et al. (2013). PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer, 60(3), 402–408. doi:10.1002/pbc.24233.
DeWalt, D. A., Gross, H. E., Gipson, D. S., Selewski, D. T., DeWitt, E. M., Dampier, C. D., et al. (2015). PROMIS((R)) pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Quality of Life Research, 24(9), 2195–2208. doi:10.1007/s11136-015-0953-3.
Walker, A. J., Johnson, K. P., Miaskowski, C., Lee, K. A., & Gedaly-Duff, V. (2010). Sleep quality and sleep hygiene behaviors of adolescents during chemotherapy. Journal of Clinical Sleep Medicine, 6(5), 439–444.
Sawyer, M., Antoniou, G., Toogood, I., & Rice, M. (1999). A comparison of parent and adolescent reports describing the health-related quality of life of adolescents treated for cancer. International Journal of Cancer, Suppl 12, 39–45.
Hockenberry-Eaton, M., Manteuffel, B., & Bottomley, S. (1997). Development of two instruments examining stress and adjustment in children with cancer. Journal of Pediatric Oncology Nursing, 14(3), 178–185.
Noll, R. B., Gartstein, M. A., Vannatta, K., Correll, J., Bukowski, W. M., & Davies, W. H. (1999). Social, emotional, and behavioral functioning of children with cancer. Pediatrics, 103(1), 71–78.
Jacobs, S., Baggott, C., Agarwal, R., Hesser, T., Schechter, T., Judd, P., et al. (2013). Validation of the Children’s International Mucositis Evaluation Scale (ChIMES) in paediatric cancer and SCT. British Journal of Cancer, 109(10), 2515–2522. doi:10.1038/bjc.2013.618.
Tomlinson, D., Gibson, F., Treister, N., Baggott, C., Judd, P., Hendershot, E., et al. (2010). Refinement of the Children’s International Mucositis Evaluation Scale (ChIMES): Child and parent perspectives on understandability, content validity and acceptability. European Journal of Oncology Nursing, 14(1), 29–41. doi:10.1016/j.ejon.2009.10.004.
Tomlinson, D., Hesser, T., Maloney, A. M., Ross, S., Naqvi, A., & Sung, L. (2014). Development and initial evaluation of electronic Children’s International Mucositis Evaluation Scale (eChIMES) for children with cancer. Supportive Care in Cancer, 22(1), 115–119. doi:10.1007/s00520-013-1953-x.
Woolery, M., Carroll, E., Fenn, E., Wieland, H., Jarosinski, P., Corey, B., et al. (2006). A constipation assessment scale for use in pediatric oncology. Journal of Pediatric Oncology Nursing, 23(2), 65–74. doi:10.1177/1043454205285874.
Hinds, P. S., Hockenberry, M., Rai, S. N., Zhang, L., Razzouk, B. I., Cremer, L., et al. (2007). Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. Journal of Pain and Symptom Management, 33(6), 686–697.
Hinds, P. S., Hockenberry, M., Tong, X., Rai, S. N., Gattuso, J. S., McCarthy, K., et al. (2007). Validity and reliability of a new instrument to measure cancer-related fatigue in adolescents. Journal of Pain and Symptom Management, 34(6), 607–618. doi:10.1016/j.jpainsymman.2007.01.009.
Hinds, P. S., Yang, J., Gattuso, J. S., Hockenberry, M., Jones, H., Zupanec, S., et al. (2010). Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. Journal of Pain and Symptom Management, 39(3), 572–578. doi:10.1016/j.jpainsymman.2009.07.015.
Mandrell, B. N., Yang, J., Hooke, M. C., Wang, C., Gattuso, J. S., Hockenberry, M., et al. (2011). Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. Journal of Pediatric Oncology Nursing, 28(5), 287–294. doi:10.1177/1043454211418667.
Bhatia, S., Jenney, M. E., Bogue, M. K., Rockwood, T. H., Feusner, J. H., Friedman, D. L., et al. (2002). The minneapolis-manchester quality of life instrument: Reliability and validity of the adolescent form. Journal of Clinical Oncology, 20(24), 4692–4698. doi:10.1200/JCO.2002.05.103.
Wu, E., Robison, L. L., Jenney, M. E., Rockwood, T. H., Feusner, J., Friedman, D., et al. (2007). Assessment of health-related quality of life of adolescent cancer patients using the Minneapolis-Manchester Quality of Life Adolescent Questionnaire. Pediatric Blood & Cancer, 48(7), 678–686. doi:10.1002/pbc.20874.
Bhatia, S., Jenney, M. E., Wu, E., Bogue, M. K., Rockwood, T. H., Feusner, J. H., et al. (2004). The minneapolis-manchester quality of life instrument: Reliability and validity of the youth form. Journal of Pediatrics, 145(1), 39–46. doi:10.1016/j.jpeds.2004.02.034.
Shankar, S., Robison, L., Jenney, M. E., Rockwood, T. H., Wu, E., Feusner, J., et al. (2005). Health-related quality of life in young survivors of childhood cancer using the minneapolis-manchester quality of life-youth form. Pediatrics, 115(2), 435–442. doi:10.1542/peds.2004-0649.
Manji, A., Tomlinson, D., Ethier, M. C., Gassas, A., Maloney, A. M., & Sung, L. (2012). Psychometric properties of the oral mucositis daily questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Supportive Care in Cancer, 20(6), 1251–1258. doi:10.1007/s00520-011-1211-z.
Tomlinson, D., Isitt, J. J., Barron, R. L., Doyle, J., Judd, P., Gassas, A., et al. (2008). Determining the understandability and acceptability of an oral mucositis daily questionnaire. Journal of Pediatric Oncology Nursing, 25(2), 107–111. doi:10.1177/1043454208314458.
Stinson, J. N., Jibb, L. A., Nguyen, C., Nathan, P. C., Maloney, A. M., Dupuis, L. L., et al. (2015). Construct validity and reliability of a real-time multidimensional smartphone app to assess pain in children and adolescents with cancer. Pain, 156(12), 2607–2615. doi:10.1097/j.pain.0000000000000385.
Morley, T. E., Cataudella, D., Fernandez, C. V., Sung, L., Johnston, D. L., Nesin, A., et al. (2014). Development of the Pediatric Advanced Care Quality of Life Scale (PAC-QoL): evaluating comprehension of items and response options. Pediatric Blood & Cancer, 61(10), 1835–1839. doi:10.1002/pbc.25111.
Seid, M., Varni, J. W., Rode, C. A., & Katz, E. R. (1999). The pediatric cancer quality of life inventory: a modular approach to measuring health-related quality of life in children with cancer. International Journal of Cancer Supplement, 12, 71–76.
Varni, J. W., Katz, E. R., Seid, M., Quiggins, D. J., & Friedman-Bender, A. (1998). The pediatric cancer quality of life inventory-32 (PCQL-32): I. Reliability and validity. Cancer, 82(6), 1184–1196.
Lai, J. S., Cella, D., Peterman, A., Barocas, J., & Goldman, S. (2005). Anorexia/cachexia-related quality of life for children with cancer. Cancer, 104(7), 1531–1539. doi:10.1002/cncr.21315.
Dupuis, L. L., Taddio, A., Kerr, E. N., Kelly, A., & MacKeigan, L. (2006). Development and validation of the pediatric nausea assessment tool for use in children receiving antineoplastic agents. Pharmacotherapy, 26(9), 1221–1231. doi:10.1592/phco.26.9.1221.
Roddenberry, A., & Renk, K. (2008). Quality of life in pediatric cancer patients: The relationships among parents’ characteristics, children’s characteristics, and informant concordance. Journal of Child and Family Studies, 17(3), 402–426. doi:10.1007/s10826-007-9155-0.
Reeve, B. B., McFatrich, M., Pinheiro, L. C., Weaver, M. S., Sung, L., Withycombe, J. S., et al. (2017). Eliciting the child’s voice in adverse event reporting in oncology trials: Cognitive interview findings from the pediatric patient-reported outcomes version of the common terminology criteria for adverse events initiative. Pediatric Blood & Cancer. doi:10.1002/pbc.26261.
Fortier, M. A., Wahi, A., Bruce, C., Maurer, E. L., & Stevenson, R. (2014). Pain management at home in children with cancer: A daily diary study. Pediatric Blood & Cancer, 61(6), 1029–1033. doi:10.1002/pbc.24907.
Parsons, S. K., Fairclough, D. L., Wang, J., & Hinds, P. S. (2012). Comparing longitudinal assessments of quality of life by patient and parent in newly diagnosed children with cancer: The value of both raters’ perspectives. Quality of Life Research, 21(5), 915–923. doi:10.1007/s11136-011-9986-4.
Penn, A., Lowis, S. P., Hunt, L. P., Shortman, R. I., Stevens, M. C., McCarter, R. L., et al. (2008). Health related quality of life in the first year after diagnosis in children with brain tumours compared with matched healthy controls; a prospective longitudinal study. European Journal of Cancer, 44(9), 1243–1252. doi:10.1016/j.ejca.2007.09.015.
Razzouk, B. I., Hord, J. D., Hockenberry, M., Hinds, P. S., Feusner, J., Williams, D., et al. (2006). Double-blind, placebo-controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. Journal of Clinical Oncology, 24(22), 3583–3589. doi:10.1200/JCO.2005.03.4371.
Varni, J. W., Burwinkle, T. M., Katz, E. R., Meeske, K., & Dickinson, P. (2002). The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer, 94(7), 2090–2106.
Wesley, K. M., Zelikovsky, N., & Schwartz, L. A. (2013). Physical symptoms, perceived social support, and affect in adolescents with cancer. Journal of Psychosocial Oncology, 31(4), 451–467. doi:10.1080/07347332.2013.798761.
Phipps, S., & Steele, R. (2002). Repressive adaptive style in children with chronic illness. Psychosomatic Medicine, 64(1), 34–42.
Schneider, S. M., & Workman, M. L. (1999). Effects of virtual reality on symptom distress in children receiving chemotherapy. Cyberpsychology & Behavior, 2(2), 125–134. doi:10.1089/cpb.1999.2.125.
O’Sullivan, C., Dupuis, L. L., Gibson, P., Johnston, D. L., Baggott, C., Portwine, C., et al. (2014). Refinement of the symptom screening in pediatrics tool (SSPedi). British Journal of Cancer, 111(7), 1262–1268. doi:10.1038/bjc.2014.445.
Redd, W. H., Jacobsen, P. B., Die-Trill, M., Dermatis, H., McEvoy, M., & Holland, J. C. (1987). Cognitive/attentional distraction in the control of conditioned nausea in pediatric cancer patients receiving chemotherapy. Journal of Consulting and Clinical Psychology, 55(3), 391–395.
Mulhern, R. K., Fairclough, D., Douglas, S. M., & Smith, B. (1994). Physical distress and depressive symptomatology among children with cancer. Children’s Health Care, 23(3), 167–179. doi:10.1207/s15326888chc2303_2.
Hinds, P. S., Hockenberry, M. J., Gattuso, J. S., Srivastava, D. K., Tong, X., Jones, H., et al. (2007). Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer, 110(10), 2321–2330. doi:10.1002/cncr.23039.
Hinds, P. S., Hockenberry, M., Rai, S. N., Zhang, L., Razzouk, B. I., McCarthy, K., et al. (2007). Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum, 34(2), 393–402. doi:10.1188/07.ONF.393-402.
Hooke, M. C., Garwick, A. W., & Gross, C. R. (2011). Fatigue and physical performance in children and adolescents receiving chemotherapy. Oncology Nursing Forum, 38(6), 649–657. doi:10.1188/11.ONF.649-657.
Hooke, M. C., McCarthy, K., Taylor, O., & Hockenberry, M. J. (2015). Fatigue and carnitine levels over multiple cycles of chemotherapy in children and adolescents. European Journal of Oncology Nursing, 19(1), 7–12. doi:10.1016/j.ejon.2014.07.015.
Hooke, M. C., Gilchrist, L., Tanner, L., Hart, N., & Withycombe, J. S. (2016). Use of a fitness tracker to promote physical activity in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer, 63(4), 684–689. doi:10.1002/pbc.25860.
Jacobs, S., Mowbray, C., Cates, L. M., Baylor, A., Gable, C., Skora, E., et al. (2016). Pilot study of massage to improve sleep and fatigue in hospitalized adolescents with cancer. Pediatric Blood & Cancer, 63(5), 880–886. doi:10.1002/pbc.25902.
Penn, A., Lowis, S. P., Stevens, M. C., Hunt, L. P., Shortman, R. I., McCarter, R. J., et al. (2009). Family, demographic and illness-related determinants of HRQL in children with brain tumours in the first year after diagnosis. Pediatric Blood & Cancer, 53(6), 1092–1099. doi:10.1002/pbc.22157.
Penn, A., Shortman, R. I., Lowis, S. P., Stevens, M. C., Hunt, L. P., McCarter, R. J., et al. (2010). Child-related determinants of health-related quality of life in children with brain tumours 1 year after diagnosis. Pediatric Blood & Cancer, 55(7), 1377–1385. doi:10.1002/pbc.22743.
Klaassen, R. J., Krahn, M., Gaboury, I., Hughes, J., Anderson, R., Grundy, P., et al. (2010). Evaluating the ability to detect change of health-related quality of life in children with Hodgkin disease. Cancer, 116(6), 1608–1614. doi:10.1002/cncr.24883.
Tyc, V. L., Fairclough, D., Fletcher, B., Leigh, L., & Mulhern, R. K. (1995). Children’s distress during magnetic resonance imaging procedures. Child Health Care, 24(1), 5–19. doi:10.1207/s15326888chc2401_2.
Wall, V. J., & Womack, W. (1989). Hypnotic versus active cognitive strategies for alleviation of procedural distress in pediatric oncology patients. American Journal of Clinical Hypnosis, 31(3), 181–191. doi:10.1080/00029157.1989.10402887.
Acknowledgements
The authors would like to thank Natalie Ernecoff, Kathryn Jackson, Susan Keller, and Sejin Lee for their help with this study.
Funding
This research was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA175759.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent from study subjects was not needed as the University of North Carolina at Chapel Hill IRB granted this research exemption from review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinheiro, L.C., McFatrich, M., Lucas, N. et al. Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review. Qual Life Res 27, 291–319 (2018). https://doi.org/10.1007/s11136-017-1692-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1692-4