Abstract
The engineering process of C4 photosynthesis into C3 plants requires an increased activity of phosphoenolpyruvate carboxylase (PEPC) in the cytosol of leaf mesophyll cells. The literature varies on the physiological effect of transgenic maize (Zea mays) PEPC (ZmPEPC) leaf expression in Oryza sativa (rice). Therefore, to address this issue, leaf–atmosphere CO2 and 13CO2 exchanges were measured, both in the light (at atmospheric O2 partial pressure of 1.84 kPa and at different CO2 levels) and in the dark, in transgenic rice expressing ZmPEPC and wild-type (WT) plants. The in vitro PEPC activity was 25 times higher in the PEPC overexpressing (PEPC-OE) plants (~20% of maize) compared to the negligible activity in WT. In the PEPC-OE plants, the estimated fraction of carboxylation by PEPC (β) was ~6% and leaf net biochemical discrimination against 13CO2\(\left( {\Delta_{\text{bio}} } \right)\) was ~ 2‰ lower than in WT. However, there were no differences in leaf net CO2 assimilation rates (A) between genotypes, while the leaf dark respiration rates (Rd) over three hours after light–dark transition were enhanced (~ 30%) and with a higher 13C composition \(\left( {\delta^{ 1 3} {\text{C}}_{\text{Rd}} } \right)\) in the PEPC-OE plants compared to WT. These data indicate that ZmPEPC in the PEPC-OE rice plants contributes to leaf carbon metabolism in both the light and in the dark. However, there are some factors, potentially posttranslational regulation and PEP availability, which reduce ZmPEPC activity in vivo.
Similar content being viewed by others
Introduction
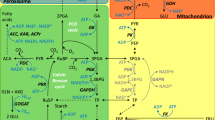
Genetic engineering of C3 plants to perform C4 photosynthesis will require overexpression of phosphoenolpyruvate carboxylase (PEPC) in the leaf mesophyll (M) cells to catalyze the initial carboxylation reaction (Gehlen et al. 1996; Häusler et al. 2002; Fukayama et al. 2003). To date, the overexpression of PEPC (PEPC-OE) in M cells of several C3 species (e.g., tobacco, potato, wheat, Arabidopsis thaliana, and rice) has shown differences in PEPC content, activity, and physiological impacts (Matsuoka et al. 2001; Häusler et al. 2002; Miyao and Fukayama 2003; Zhu et al. 2010; Qi et al. 2017). The presence of transgenic C4-type PEPC activity in rice enhanced the stomatal and mesophyll CO2 conductances (gsC and gm, respectively) and improved leaf net CO2 assimilation rates (Ku et al. 1999, 2000; Lian et al. 2014). Alternatively, other rice PEPC-OE studies showed decreases in leaf net CO2 assimilation compared to wild-type (WT), particularly under low photorespiratory conditions. This result was suggested to occur because of reduced ribulose-1,5-bisphosphate carboxylase:oxygenase (Rubisco) activity (Fukayama et al. 2003; Taniguchi et al. 2008), and enhanced rates of leaf mitochondrial non-photorespiratory CO2 release in the light (RL) (Miyao 2003). Higher RL in the PEPC-OE plants is consistent with the anaplerotic function of PEPC (Jeanneau et al. 2002; O’Leary et al. 2011; Abadie and Tcherkez 2018) and a stimulation of the tricarboxylic acid (TCA) cycle with the increased PEPC activity (Agarie et al. 2002; Fukayama et al. 2003; Kandoi et al. 2016). In addition, a decrease in O2 sensitivity in PEPC-OE rice plants was reported (Agarie et al. 2002; Fukayama et al. 2003). Collectively, the increase in PEPC activity in C3 plants has provided inconsistent effects on leaf photosynthesis, despite indications that the transferred PEPC gene can produce a functional enzyme (Fukayama et al. 2003). In addition, subtle changes in leaf CO2 assimilation may not be detected with traditional leaf gas exchange measurements.
However, the combined analysis of leaf–atmosphere CO2 exchange and discrimination against 13CO2 can be a useful tool to gain mechanistic insights into changes in leaf carbon metabolism in the light (Farquhar et al. 1989; von Caemmerer 1989; Ghashghaie et al. 2003; Cousins et al. 2007; von Caemmerer et al. 2014). For example, in C3 plants (instantaneous) leaf net discrimination against 13CO2 in the light (∆o, ‰) is determined by the discrimination against 13CO2 during CO2 movement from the atmosphere to the chloroplast stroma of M cells, and the 13CO2 discriminations associated with carboxylation, photorespiration, and mitochondrial non-photorespiratory respiration (von Caemmerer and Evans 1991; Le Roux et al. 2001; Barbour et al. 2010; Bickford et al. 2010; Tazoe et al. 2011; Evans and von Caemmerer 2013). The 13CO2 fractionation due to the carboxylation reactions (b, ‰) includes the contributions of both Rubisco and PEPC (Cernusak et al. 2013; Ubierna and Farquhar 2014), where the 13CO2 fractionation by Rubisco (b3) is ~29.0‰ with respect to dissolved CO2 (Roeske and O’Leary 1984) and is typically assumed to be fairly constant across species and insensitive to temperature (Ghashghaie et al. 2003; Evans and von Caemmerer 2013). By contrast, the net 13CO2 fractionation associated with PEPC (b4) is circa − 5.7‰ at 25 °C, which includes the 13C fractionations during CO2 dissolution in water, catalyzed hydration of CO2 to bicarbonate (HCO3−) and PEP carboxylation (von Caemmerer et al. 2014). In C3 plants, b for gaseous CO2 ranges between 28.2 and 30‰, corresponding to carboxylation by PEPC from 5% of total leaf carbon uptake to zero, respectively (Brugnoli et al. 1988; Gillon and Griffiths 1997; Ghashghaie et al. 2003). When the contribution by PEPC activity to total carbon uptake rate is large, for example, in the C3–C4 intermediate Flaveria floridana where PEPC activity was estimated to be 12 to 20% of the total carboxylation, this can significantly affect ∆o (Alonso-Cantabrana and von Caemmerer 2016). This impact of PEPC activity on ∆o provides a benchmark for evaluating the contribution of C4-PEPC (e.g., from Zea mays, ZmPEPC) activity to carbon uptake in rice.
However, in addition to the carboxylation reactions, photorespiration and mitochondrial non-photorespiratory respiration can also influence ∆o. For example, ∆o decreases at a given ratio of intercellular: atmospheric CO2 partial pressures (Ci/Ca) with the discrimination against 13CO2 associated with photorespiration (∆f). By contrast, discrimination against 13CO2 associated with non-photorespiratory mitochondrial respiration (∆e) may decrease or increase ∆o depending on the offset in 13CO2 compositions \(\left( {\delta^{ 1 3} {\text{C}},\permille} \right)\) between leaf chamber during measurements and growth chamber (Gillon and Griffiths 1997; Ghashghaie et al. 2003; Gong et al. 2015). The absolute 13CO2 fractionation due to mitochondrial non-photorespiratory decarboxylating reactions (e, ‰; relative to photosynthetic products) is usually considered to be lower than the 13CO2 fractionation of photorespiration (f, ‰; Rooney 1988; Ivlev et al. 1996; Tazoe et al. 2009; Wingate et al. 2007). However, the magnitude of ∆e (‰) depends on e, the 13CO2 signature of the substrates used for respiration (Stutz et al. 2014) and the ratio of RL to the carboxylation rate. The leaf net discrimination against 13CO2 leads to photosynthetic products depleted in the heavier carbon isotope compared to atmospheric CO2, and since ∆o and the carbon pool used by leaf respiration change during the day (Tcherkez et al. 2017a), it is difficult to accurately estimate \(\delta ^{{13}} {\text{C}}\) of RL substrates. In addition, estimates of RL are indirect, and it has been shown that mitochondrial respiration activity is partially inhibited in the light (Tcherkez et al. 2005). There are also indications that photorespiration plays a role in regulating the degree of RL inhibition (Abadie et al. 2017; Tcherkez et al. 2017a; Gauthier et al. 2018). Alternatively, rates of leaf CO2 evolution in the dark (Rd) and \(\delta^{ 1 3} {\text{C}}\) of dark-respired CO2\(\left( {\delta^{ 1 3} {\text{C}}_{\text{Rd}} ,\permille} \right)\) can be directly measured, where \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) largely depends on the \(\delta^{ 1 3} {\text{C}}\) of substrates feeding respiration, particularly the pool of leaf carbon previously produced in the light (Atkin et al. 1998; Barbour et al. 2007; Lehmann et al. 2016a, b; Gessler et al. 2017). Higher Rd values in PEPC overexpressing plants have been observed on potato (Gehlen et al. 1996; Häusler et al. 1999; Rademacher et al. 2002) and also rice (Agarie et al. 2002). Therefore, combined analysis of the leaf CO2 evolution flux in the dark (Rd) and of \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) may provide useful insights into leaf carbon metabolism in PEPC-OE plants versus WT in the light.
The purpose of the current study was to quantify the effects of transgenic expression of ZmPEPC on leaf–atmosphere CO2 exchange and discrimination against 13CO2 in Oryza sativa, with particular interest on the net biochemical 13CO2 fractionation due to the carboxylating enzymes (∆bio). Measurements were made under low photorespiratory conditions (O2 partial pressure of 1.84 kPa) to minimize the contribution of photorespiration and refixation of photorespired CO2 to leaf net discrimination against 13CO2. In addition, three atmospheric CO2 partial pressures (pCO2) were used (18.4, 35.0, and 92.1 Pa) to potentially manipulate the relative contributions of PEPC and Rubisco to carboxylation. Leaf net biochemical discrimination against 13CO2 (Δbio, ‰), which includes both Rubisco and PEPC fractionations, was used to estimate the in vivo contribution to carboxylation by PEPC (β) in the PEPC-OE plants. Leaf–atmosphere CO2 and 13CO2 exchanges were also measured in the dark to determine Rd and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) to further explain the effect of ZmPEPC to leaf carbon metabolism.
Materials and methods
Plant material
Generation of ZmPEPC-expressing transgenic rice lines
Generation of transgenic Oryza sativa (rice) lines expressing maize (Zea mays) phosphoenolpyruvate carboxylase (ZmPEPC) was done at the International Rice Research Institute (IRRI; Los Baños, Philippines). Agrobacterium tumefaciens (strain LBA4404)-mediated transformation was performed following the method described by Hiei and Komari (2006). A pSC0/ZmPEPC vector (see Supplementary material, Fig. S1A) containing a full-length genomic fragment (GenBank Accession no. AF234296.1) was created by subcloning ZmPEPC from pIG121Hm/ZmPEPC (from Mitsue Miyao, National Institute of Agrobiological Sciences, Tsukuba, Japan; Ku et al. 1999) into pSC0 (GenBank, Accession no. KT365905; Lin et al. 2016) using a SmaI restriction digest. Expression of ZmPEPC is driven by its native promoter and terminator. A co-transformation of pSC0/ZmPEPC with pCAMBIA1300, a binary vector with the hygromycin B resistance gene, was performed for selection. Freshly harvested immature embryos of rice (Oryza sativa spp. indica cv. IR64) 8–12 days after anthesis were used as explants. After one week of co-cultivation in Murashige and Skoog medium and resting for 5 days, emerging resistant calli were selected with 50 mg L−1 of hygromycin B. A total of 83 transgenic rice plantlets regenerated from hygromycin-resistant calli were kept in hydroponics (Yoshida culture solution; Yoshida et al. 1972) for 2 weeks to acclimate. A total of 59 ZmPEPC PCR positive plants from these lines were grown in soil. Plants with a single copy of the transgene and more than 50% ZmPEPC protein accumulation relative to the maize control were advanced to further generations to obtain homozygous lines. At T3 generation, homozygosity was confirmed on four plants (events) by DNA blot analysis for T-DNA integration (see Fig. S1B for PEPC-28 event). All four events had abundant ZmPEPC accumulation relative to maize as detected by immunoblot (see Fig. S2A). Progeny of the PEPC-28 event showed that detectable ZmPEPC localizes to rice M cells (Fig. S2B), and the subsequent T4 generation (line PEPC-28) was chosen for analysis in the present study. Oryza sativa cv. IR64 line A009 was used as a negative control for transgene expression, and Zea mays cv. B73 was used as a positive control for protein accumulation throughout.
Plant growth conditions
PEPC-OE (line PEPC-28) and WT plants (cv. IR64, line A009) were grown, together with Zea mays cv. B73 plants, in a controlled environment growth chamber (Gch; Bigfoot series, BioChambers Inc., Winnipeg, MB, Canada) at the School of Biological Sciences at Washington State University, Pullman, WA (USA). Plants were individually grown in 4-L free drainage pots as described in Giuliani et al. (2013). The photoperiod was 14 h, from 8:00 to 22:00 h standard time. Light was provided by F54T5/841HO Fluorescent 4100 K and 40 W Halogen incandescent bulbs (Philips) and was supplied in a bell-shaped pattern during the photoperiod with a maximum photosynthetic photon flux density (PPFD) of 600 mol photons m−2 s−1 incident on the plant canopy for 10 h. Air temperature was 22 °C in the dark, and after switching on the light, it tracked the PPFD pattern with a maximum of 26 °C for 10 h. Air relative humidity was maintained at ~70% so that the maximum air Vapor Pressure Deficit (VPD) was ~1.6 kPa. During the photoperiod, the atmospheric pCO2 in the Gch was enriched with CO2 supplied by a pressurized tank and maintained at 184.2 Pa (2000 μmol CO2 mol−1 air); the 13CO2 composition \(\left( {\delta^{ 1 3} {\text{C}}_{\text{Gch}} } \right)\) was − 41.6‰ (± 0.1 SE; n = 8) determined as in Giuliani et al. (2019).
Leaf biochemical analysis
The percentage of PEPC in PEPC-OE and WT rice compared to maize was determined by protein immunoblot technique according to Koteyeva et al. (2015). In the two rice plant types and in maize, the in vitro activities of PEPC and Rubisco per unit leaf surface area (μmol m−2 s−1) were determined spectrophotometrically as described by Cousins et al. (2007) and Walker et al. (2013), respectively. Leaf malate content (mmol malate m−2) was determined spectrophotometrically based on the method of Hatch (1979) and Edwards et al. (1982). These methods for leaf biochemical analysis are reported in Method S1.
Leaf physiological analysis
System set-up for coupled measurements of leaf–atmosphere CO2, H2O, and 13CO2 exchanges
Measurements were performed in Pullman, WA (mean atmospheric pressure of 92.1 kPa). Two LI-6400XT portable gas analyzers (LI-COR Biosciences, NE, USA; detecting 12CO2) operating as an open system were coupled to a tunable-diode laser absorption spectroscope, which detects 12CO2 and 13CO2 isotopologs (TDLAS model TGA200A, Campbell Scientific, Inc., Logan, UT, USA). The system set-up was as described in Giuliani et al. (2019), based on Ubierna et al. (2013), Stutz et al. (2014), and Sun et al. (2014).
Leaf photosynthesis was determined with a LI-COR equipped with a 2 × 3 cm2 leaf chamber (Lch) and a 6400-02B LED light source (LI-COR Biosciences). Alternatively, leaf dark respiration was determined with an 8 × 10 cm2 custom-built Lch having an adaxial glass window, and with a volume of ~ 100 cm3 (Barbour et al. 2007, based on Sharkey et al. 1985). The chamber had a hollowed stainless-steel frame sealed with a closed-cell foam gasket and was connected to a circulating water bath for temperature control in the lumen. Before the dark respiration measurements, the leaf portion in the Lch was illuminated with a 6400-18 RGB light source (LI-COR Biosciences).
Protocol for coupled measurements of leaf–atmosphere CO2, H2O, and 13CO2 exchanges
Leaf photosynthetic measurements (n = 4 in PEPC-OE and n = 5 in WT) were taken between 9:00 and 16:00 h standard time; on each plant, the mid-to-distal portions of two fully expanded leaves from the same stem were positioned to completely cover the Lch section. Measurements were taken at atmospheric CO2 partial pressures in the Lch (Ca) of 18.4, 35.0, and 92.1 Pa (i.e., 200, 380, and 1000 µmol CO2 mol−1 air, respectively) and with 13CO2 composition entering the Lch (δin, corresponding to the 13C composition of the CO2 source from a pressurized tank) of − 48.0‰. The O2 partial pressure (pO2) was set at 1.84 kPa (i.e., 20 mmol O2 mol−1 air), PPFD was 1500 µmol photons m−2 s−1, tleaf was 25 °C, and leaf-to-air VPD was kept in the range of 1.0–1.5 kPa. The airflow rate through the LI-COR system was 300 µmol s−1 (~ 0.48 L min−1), and gas analyzers were matched after each change in Ca when the TDLAS was not measuring the air leaving the Lch. Leaves were acclimated for about 30 min, and the data were recorded for an additional 30–40 min under each measurement condition. The rate of net CO2 assimilation per leaf surface area (A, µmol CO2 m−2 s−1), stomatal conductance to CO2 diffusion (gsC, µmol CO2 m−2 s−1 Pa−1), intercellular pCO2 (Ci, Pa), and the ratio Ci:Ca (Ci/Ca) were determined. The 13C signature of leaf dry matter (δ13Cdm, ‰) and total N content as fraction (%) of leaf dry matter (n = 4 for PEPC-OE; n = 5 for WT) were determined by isotope ratio mass spectrometry (IRMS) as described in Giuliani et al. (2019). Total N content per unit leaf surface area (g m−2) was then calculated based on leaf dry matter per area.
Leaf dark respiration measurements were performed on two plants per day (one PEPC-OE and one WT). Each plant was taken out of the Gch at 9:30 h standard time, and the mid-to-distal portions of 8–9 fully expanded leaves, similar to those used for the photosynthetic analysis, were enclosed in the custom-built Lch to completely cover the section area of ~ 76 cm2. Under pO2 of 1.84 kPa, the leaves were first exposed to 750 µmol photons m−2 s−1 of PPFD for 20 min, 500 for 15 min (at tleaf of 25 °C), and 100 µmol photons m−2 s−1 for 5 min (at tleaf of 30 °C). The airflow rate through the LI-COR system was changed from 700, to 500, and to 350 µmol s−1 tracking the decreasing PPFD. The measurement CO2 (supplied by a new cartridge every day) had \(\delta^{ 1 3} {\text{C}}\) from − 6.2 to − 4.8‰ to generate a large \(\delta^{ 1 3} {\text{C}}\) difference between the Lch and the Gch atmosphere (− 41.6‰). The Ca in the Lch was maintained at 35.0 Pa. The stepwise decrease in PPFD and airflow rate minimized the perturbation to the gas exchange measurements when transitioning the leaf from the light to dark. After 40 min of leaf photosynthesis, dark was imposed in the Lch, and leaf CO2 evolution was measured at pO2 of 18.4 kPa and tleaf of 30 °C for 195 min to determine the dynamics of the dark respiration rate per unit (one side) leaf surface area (Rd, µmol CO2 m−2 s−1) and corresponding \(\delta^{ 1 3} {\text{C}}\)\(\left( {\delta^{ 1 3} {\text{C}}_{\text{Rd}} ,\permille} \right)\) (n = 4). The tleaf was set at 30 °C to enhance the precision of the dark measurements, and the gas analyzers were matched at the beginning of the dark period and every 15 min thereafter, when the TDLAS was not measuring the leaf chamber air. In addition, three plants (n = 3) of the PEPC-OE line and of WT were taken out of the Gch at 12:00 h 3 days after their use for photosynthesis measurements and darkened at 25 °C for 24 h. Subsequently, leaf dark CO2 evolution was measured at tleaf of 30 °C and at pO2 of 18.4 Pa to determine Rd(24h) (µmol CO2 m−2 s−1) and \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}( 2 4 {\text{h}})}} \left( \permille \right)\). The description of the abbreviations, and symbol and unit of the environmental parameters and leaf variables are listed in Table S1.
The net discrimination against 13CO2 in the light, mesophyll CO2 conductance, and 13C composition of dark-evolved CO2
Instantaneous leaf net discrimination against 13CO2 in the light (∆o, ‰) was calculated by mass balance according to Evans et al. (1986). The leaf net biochemical discrimination against 13CO2 (∆bio, ‰), which depends on the biochemistry of net CO2 uptake, was determined for the PEPC-OE plants at the different Ca (using Eq. S1 in Method S2), where the mesophyll conductance to CO2 diffusion (gm, μmol CO2 m−2 s−1 Pa−1) is a required input (Alonso-Cantabrana and von Caemmerer 2016). In the applied procedure, ∆bio and gm are not independent variables), and ∆bio is a proxy of b (the in vivo 13CO2 carboxylation fractionation; see Eq. 1 below) that is necessary to determine gm. The ∆bio in PEPC-OE plants were therefore calculated assuming WT gm values, which were estimated according to Evans and von Caemmerer (2013) as described in Method S3. Specifically, the mean gm values determined on the WT plants at Ca of 18.4, 35.0, and 92.1 Pa were used to calculate ∆bio (n = 4) in PEPC-OE plants at the three Ca values. The assumption of equal gm between PEPC-OE and WT plants was supported by the sensitivity analysis of ∆bio on gm (see Fig. S3) compared with the ∆bio analysis in Alonso-Cantabrana and von Caemmerer (2016) for a C3–C4 intermediate species. In addition, comparable gm values were determined on the PEPC-OE and WT plants from measurements of leaf–atmosphere oxygen (in alternative to carbon) isotope exchange (see Table S2; based on Ubierna et al. 2017; Sonawane and Cousins 2018). However, leaf 18O based gm is not strictly associated with the biochemistry of photosynthesis as is the 13C based gm, and therefore, it could not be used in the present analysis to determine ∆bio in PEPC-OE plants.
In PEPC-OE plants, the fraction of carboxylation by PEPC (β, mol C(by_PEPC) mol−1 C(by_Rubisco+PEPC); n = 4) was determined by solving for the β value that minimized the difference between the ∆bio determined by eq. S1 and ∆bio modeled (∆bio_mod, ‰) based on Griffiths et al. (2007) as
assuming the 13CO2 fractionation of Rubisco (b3) and PEPC (b4) were 29.0 and − 5.7‰, respectively. The RL (µmol CO2 m−2 s−1) is mitochondrial non-photorespiratoy respiration rate in the light; e* (‰) is the experimental 13CO2 fractionation associated with RL, and the term (A + RL) corresponds to the carboxylation rate by Rubisco plus PEPC. Under the assumption that at the same tleaf, there was no difference between leaf mitochondrial respiration rate in the light (RL) and three hours after light–dark transition (Rd(3h), µmol CO2 m−2 s−1; Table 3), RL at tleaf of 25 °C was predicted from Rd(3h) at 30 °C using the temperature response function in Bernacchi et al. (2001). The e* (− 6.4‰) was determined as the difference between \(\delta^{ 1 3} {\text{C}}\) of the CO2 entering the leaf chamber during photosynthetic measurements (− 48‰) and in the growth chamber (− 41.6‰).
The term \(\left[ {b_{3} - \beta \left( {b_{3} - b_{4} } \right)} \right]\varvec{ }\) in Eq. 1 is equivalent to the in vivo 13CO2 carboxylation fractionation (b, ‰), as reported in Farquhar and Richards (1984). Therefore, b can be calculated for the PEPC-OE plants using β estimated as described above. Alternatively, ∆bio_mod can be predicted in WT assuming b is equal to b3 (29.0‰), i.e., no b4 and β values were applied given a negligible in vitro PEPC activity in WT plants (see “Results”).
Leaf net discrimination against 13CO2 in the light for PEPC-OE and WT plants was predicted based on Ubierna and Farquhar (2014) with a simplified equation as
where a = 4.4‰ is the 13CO2 fractionation during CO2 diffusion through stomata; b = [b3 − β(b3–b4)] for PEPC-OE (Eq. 1; see Results) and b = 29‰ for WT; Cc is the pCO2 in the chloroplast (Pa), as calculated by Fick’s first law as Cc = Ci − A/gm.
The 13C composition in leaf respired CO2 in the dark \((\delta^{ 1 3} {\text{C}}_{\text{Rd}} ,\permille)\) was calculated according to Barbour et al. (2007), based on Evans et al. (1986).
Given \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}(i)}}\) as the value of \(\delta^{ 1 3} {\text{C}}\) for dark-evolved CO2 at time i (by 3 min over 195 min from light–dark transition), the fractional contribution of Lch carbon assimilates to \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}(i)}}\)\(\left( {^{{\delta {\text{Rd}}}} L_{{{\text{ch}}\_{\text{substr}}(i)}} ,{\permille \mathord{\left/ {\vphantom {\permille \permille}} \right. \kern-0pt} \permille}} \right)\) was calculated for PEPC-OE and WT plants (n = 4) according to Giuliani et al. (2019) as
where \(\delta^{13} C_{{{\text{Rd}}\left( {24{\text{h}}} \right)}}\) are as given in Table 3, and \(\delta^{13} C_{{{\text{Lch}}\_{\text{Ph}}}} \left( \permille \right)\) are the representative \(\delta^{ 1 3} {\text{C}}\) of PEPC-OE or WT carbon assimilates produced in the Lch before light–dark transition. The assumptions underlying Eq. 3 are described in Giuliani et al. (2019), and the values of the variables used for calculations are presented in Table S3. Based on the total fractional contributions of Lch and Gch assimilates to \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) equal 1, the complementing fractional contribution of Gch assimilates to \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}(i)}} \left( {^{{\delta {\text{Rd}}}} {\text{G}}_{{{\text{ch}}\_{\text{substr}}(i)}} , \, {\permille \mathord{\left/ {\vphantom {\permille \permille}} \right. \kern-0pt} \permille}} \right)\) was determined for both plant types as \({}^{{^{{\delta {\text{Rd}}}} }}{\text{G}}_{{{\text{ch}}\_{\text{substr}}\left( i \right)}} = 1 - {}^{{^{{\delta {\text{Rd}}}} }}{\text{L}}_{{{\text{ch}}\_{\text{substr}}\left( i \right)}}\).
Leaf A–Ci response curves
For PEPC-OE and WT plants, A-Ci response curves (n = 4) were determined with the LI-6400XT through stepwise decreases in Ca from 35.0 to 3.7 Pa, at 1.84 kPa pO2, PPFD of 1500 µmol photons m−2 s−1, tleaf at 25 °C, and VPD between 1.0 and 1.5 kPa. For each response curve, a least square regression analysis was applied to the initial slope (for Ci ≤ 9.2 Pa) to calculate the CO2 compensation point (Γ, Pa).
Statistical analysis
Statistical analyses for the effects of plant-type (PEPC-OE and WT) and/or Ca level on the leaf photosynthetic and dark respiration variables are described in Method S4. In addition, a nonlinear model with three parameters was used to fit the Rd and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) responses for the two plant types over a 3-hour interval. The significance between the two plant types for the Rd or \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) responses was inferred from the analysis of the model parameters, i.e., range (difference between the initial value and the lower asymptote), exponential rate of change, and lower asymptote (floor of the response) (see Methods S4 for the description of the procedure).
Results
Leaf biochemical analysis
PEPC content and activity
Mean PEPC content in PEPC-OE and WT plants compared to Z. mays were 65% ± 2.2 SE and 4.1% ± 0.4 SE, respectively (Fig. 1a; n = 2). The in vitro mean activities of PEPC in young (expanding) leaves of PEPC-OE and WT plants were 52.3 and 4.1 µmol HCO3− m−2 s−1, respectively, and 54.9 and 2.2 µmol HCO3− m−2 s−1 in mature leaves, respectively. The in vitro mean activity of PEPC in mature Z. mays leaves was 280 µmol HCO3− m−2 s−1, and thus the PEPC activity in O. sativa PEPC-OE mature leaves was approximately 25 times greater than WT but ~ 5 times lower than that in Z. mays (Fig. 1b; n = 3). The mean ratios of in vitro PEPC: Rubisco activity in mature leaves were 0.79 ± 0.07 SE and 0.04 ± 0.01 SE for PEPC-OE and WT plants, respectively (n = 3).
a Immunoblot analysis for PEPC from soluble proteins extracted from mature rice leaves, showing protein molecular weight (kDa) and band intensity quantitation. The levels of PEPC for both PEPC-OE and WT are mean percentage values of Z. mays (n =2). b In vitro PEPC activity determined in both young and mature leaves of PEPC-OE and WT, and mature leaves of Z. mays plants. Values are mean ± SE (n = 3)
Leaf malate content
The mean malate contents per unit leaf surface area (mmol malate m−2) determined on leaf samples taken immediately after leaf photosynthetic measurements were 0.60 ± 0.13 SE in PEPC-OE and 0.55 ± 0.06 SE in WT plants, but not statistically different between plant types (n = 5; P > 0.05).
Leaf physiological analysis
Leaf photosynthetic responses
There was a significant pCO2 effect on A, gsC, Ci, Ci/Ca, Cc, Cc/Ca, and ∆o, but these parameters did not differ between PEPC-OE and WT plants (Table 1). In WT plants, gm significantly decreased with pCO2 (Table 2).
A significant plant-type effect was determined on ∆bio (‰), with lower values in PEPC-OE plants with respect to WT (Fig. 2a; P = 0.006), and on b (‰), with lower values in the PEPC-OE plants compared to a b of 29.0 in WT plants (Fig. 2a; P = 0.003). In addition, the fraction of carboxylation by PEPC (β) in the PEPC-OE plants was significantly different from β equal to 0 in WT (Fig. 2b; P < 0.001). The values of ∆bio and b tended to be lower, and β values greater, at Ca of 18.4 Pa compared to the higher pCO2, but there was not a significant effect of pCO2 or a plant-type × pCO2 effect on these parameters. For PEPC-OE and WT plants (n = 4), the overall means of ∆bio across the three experimental pCO2 range were 27.1 ± 0.5 SE and 29.2 ± 0.5 SE, respectively. In addition, b overall mean of 26.9 ± 0.5 SE and β overall mean of ~ 0.06 mol C(by_PEPC) mol−1 C(by_Rubisco+PEPC) ± 0.01 SE were calculated for the PEPC-OE plants.
a The net 13C biochemical discrimination (∆bio; triangles) and the in vivo 13CO2 carboxylation fractionation (b; circles) in the PEPC-OE (empty symbols) and WT (full symbols) plants at Ca of 18.4, 35.0, and 92.1 Pa. For WT plants ∆bio = ∆bio_mod and b equal to 29.0‰. b The carboxylation by PEPC (β) in PEPC-OE plants (empty symbol) compared to β equal to zero in WT (full symbol) at Ca of 18.4, 35.0, and 92.1 Pa. In both plots, symbols correspond to mean value ± SE (for PEPC-OE: n = 3 at Ca of 35.0 Pa, n = 4 at Ca of 18.4 and 92.1 Pa; n = 4 for WT)
The ∆13Cmod values for WT and PEPC-OE plants significantly fit the corresponding ∆o values plotted versus Cc/Ca, with slopes of 24.6‰ in WT (R2 = 0.93; P < 0.001) and 22.5‰ (R2 = 0.87; P < 0.001) in PEPC-OE plants (Fig. 3). The ∆o plotted versus Cc/Ca was generally higher for the WT compared to PEPC-OE plants (Fig. 3), and the regression line fitting ∆o in the two plant types showed a significantly higher slope in WT (24.8‰) compared to PEPC-OE (23.1‰) plants (P < 0.001; See Method S4 for statistical analysis).
Leaf net discrimination against 13CO2 in the light (∆o) versus ratio of chloroplast to atmosphere CO2 partial pressures (Cc/Ca) determined on individual PEPC-OE and WT plants (n = 4) at Ca of 18.4, 35.0, and 92.1 Pa under pO2 of 1.84 kPa. Closed symbols are for WT, and open symbols are for PEPC-OE plants; squares for Ca = 18.4, circles for Ca = 35.0 and triangles for Ca = 92.1 Pa. Lines represent the leaf net discrimination against 13CO2 predicted as ∆13Cmod = a + (b-a) × Cc/Ca (based on Ubierna and Farquhar 2014) where b = 29.0‰ for WT (solid line) and b = 26.9‰ for PEPC-OE (dashed line); a equal to 4.4‰
There was not a significant plant-type effect on \(\delta^{ 1 3} {\text{C}}\) of leaf biomass (\(\delta^{ 1 3} {\text{C}}_{\text{dm}}\); Table 3); in addition, leaf nitrogen content (g m−2) was not statistically different between PEPC-OE and WT plants: 2.0 ± 0.2 SE and 2.5 ± 0.2 SE (n = 4), respectively. Furthermore, the CO2 compensation point (\(\varGamma\), Pa) was not significantly different between transgenic and WT plants, with mean values of 0.70 ± 0.09 SE and 0.61 ± 0.07 SE (n = 4), respectively.
Leaf dark respiration responses
Before the light–dark transition, the A values at pO2 of 1.84 kPa and PPFD of 750 µmol photons m−2 s−1 were similar in the PEPC-OE and WT plants, with mean rates of 13.9 ± 0.6 SE and 14.1 ± 0.1 SE µmol CO2 m−2 s−1, respectively (n = 3). After the light–dark transition, Rd in the PEPC-OE and WT plants had a hyperbolic decrease over the 3-hour interval, with a rapid decline in the first hour (Fig. 4a). The PEPC-OE plants showed higher Rd (~25% enhanced rates; see Fig. 4a; Table 3) compared to WT over the three-hour period, with a statistical significance determined based on the analysis of the three-parameter nonlinear model selected to fit the Rd responses. Specifically, a significantly greater Rd lower asymptote was estimated in the PEPC-OE versus WT plants (µmol CO2 m−2 s−1; P < 0.0001), while Rd ranges (µmol CO2 m−2 s−1; P = 1.000) and Rd exponential rates of change (µmol CO2 m−2 s−1 min−1; P = 0.114) did not differ between the two plant types (Table S4). The mean values of RL inferred from Rd(3h), were 0.76 ± 0.05 SE for PEPC-OE and 0.56 ± 0.03 SE µmol CO2 m−2 s−1 for WT plants.
a Dynamics of leaf dark respiration rate (Rd) and b13C composition associated with Rd\(\left( {\delta^{ 1 3} {\text{C}}_{\text{Rd}} } \right)\) estimated on PEPC-OE (open circles) and WT (closed circles) after leaf exposure to light at pO2 of 1.84 kPa. Symbols in a and b correspond to mean value calculated every three min ± SE (n = 4)
In the transgenic and WT plants, \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) after the light–dark transition showed a negative hyperbolic pattern over the three-hour interval, with most of the variations occurring in the first 30 min, and with higher \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) in the PEPC-OE plants compared to WT over the entire dark period (Fig. 4b). The \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) response in the PEPC-OE plants was found to be statistically higher than that in WT plants based on the analysis of the three-parameter nonlinear model selected to fit the \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) values. In particular, the \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) ranges were not significantly different (‰; P = 0.157), but the \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) floor value was significantly higher (‰; P = 0.003) in the PEPC-OE versus WT plants (Table S5). However, the values of \(\delta^{ 1 3} {\text{C}}_{{( 6 {\text{min}})}} \,{\text{and}}\,\delta^{ 1 3} {\text{C}}_{{( 3 {\text{h}})}}\) were not significantly different between plant types (Table 3). After 24 h in the dark, there were no significant differences in Rd(24h) and \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}( 2 4 {\text{h}})}}\) between PEPC-OE and WT plants.
Discussion
Leaf photosynthetic traits in PEPC-OE versus WT plants
In the current study, the in vitro leaf activity of PEPC was ~ 25 times higher in the PEPC-OE plants than that in WT, and the mean PEPC activity relative to Rubisco activity was 79% in the transgenic plants compared to 4% in WT. However, the higher in vitro PEPC activity in the PEPC-OE plants had no detectable effect on A, or other photosynthetic parameters (e.g., stomatal conductance). Previous studies have also shown no enhancement of A in transgenic rice and tobacco expressing ZmPEPC, compared to untransformed plants, even though there was a large increase in in vitro PEPC activity (Taniguchi et al. 2008; Hudspeth et al. 1992). While other studies showed that transgenic rice plants expressing ZmPEPC had a lower O2 sensitivity of A compared to WT, due to a decrease in A at low O2 level (Ku et al. 1999, 2000; Agarie et al. 2002; Fukayama et al. 2003), other studies showed that under photorespiratory conditions A was higher in transgenic rice expressing ZmPEPC (Jiao et al. 2002) or C4 PEPC from sugarcane (Lian et al. 2014). Moreover, transgenic Arabidopsis thaliana expressing ZmPEPC with a tenfold increase of in vitro PEPC activity showed ~18% higher A compared to control plants (Kandoi et al. 2016); similar enhancement of A was also seen in ZmPEPC transgenic wheat (Hu et al. 2012). These different responses to higher C4 PEPC activities in transgenic plants of rice and other C3 species highlight the need for further research to clarify the physiological impacts as well as the control of C4-PEPC (and in particular ZmPEPC) activity in rice leaves.
There are several factors that could limit in vivo C4-PEPC activity expressed in a C3 plant. For example, 3-phosphoglyceric acid (3-PGA) produced by Rubisco could be used for synthesis of PEP to drive additional PEPC reactions to produce oxaloacetate (OAA) and then malate through malate dehydrogenase (MDH). However, the export of PEP from the chloroplast in the cytosol may limit ZmPEPC in rice (Taniguchi et al. 2008; Weber and von Caemmerer 2010). Moreover, ZmPEPC in rice is known to be in a dephosphorylated status during the light time, and therefore it operates at reduced rates because of the low affinity for PEP and the feedback (allosteric) inhibition by various metabolites such as malate, aspartate, and glutamate (Vidal and Chollet 1997; Jeanneau et al. 2002). This posttranslational PEPC control is involved in mediating carbon–nitrogen interactions; specifically, forms of PEPC with diminished feedback inhibition may increase carbon flux into organic acids (OAA and malate) and amino acids at the expense of starch and soluble sugars (Rademacher et al. 2002; O’Leary et al. 2011). In two previous studies, a 1.5- to 3-fold increase in leaf malate occurred in the light of transgenic tobacco, potato and rice overexpressing PEPC (Hudspeth et al. 1992; Häusler et al. 1999; Rademacher et al. 2002; Ku et al. 2000; Agarie et al. 2002). However, in the current study on rice, the malate content was not significantly higher in PEPC-OE plants compared to WT. The accumulation of leaf malate during the photoperiod in PEPC-OE rice plants will depend on relative rates of malate synthesis via PEPC, rates of catabolism of malate by mitochondria, and rates of export of malate outside the leaves. In general, the net rate of leaf CO2 assimilation incorporates the CO2 fluxes through carboxylation, photorespiration, and light respiration, which are difficult to disentangle with traditional measurements of leaf CO2 exchange. However, as discussed below, the combined analysis of leaf–atmosphere CO2 exchange and discrimination against 13CO2 can be a useful tool to gain insights into these various fluxes of CO2 within the leaf.
Leaf net discrimination against 13CO2 in the light, and net biochemical 13CO2 discrimination in the PEPC-OE plants
In C3 plants, leaf net discrimination against 13CO2 in the light (∆o, ‰) integrates the discrimination against 13CO2 during CO2 diffusion (in both gas and liquid phases) and due to the carboxylation and decarboxylation reactions. In particular, the Rubisco 13CO2 fractionation (b3) is 29‰, whereas the PEPC net 13CO2 fractionation associated with bicarbonate fixation (b4) is − 5.7‰ (Ubierna and Farquhar 2014; von Caemmerer et al. 2014). Therefore, an increase of the carboxylation by PEPC (β) would lower the in vivo 13CO2 carboxylation fractionation (b) and potentially decrease ∆o (Farquhar and Richards 1984; Lanigan et al. 2008; Bickford et al. 2010). In the present study, the values of b in the WT were set equal to 29.0‰ at all CO2 levels, i.e., there was no carboxylation by rice native PEPC given the negligible in vitro PEPC activity determined in WT plants. The leaf net biochemical discrimination against 13CO2 (∆bio) and b were significantly lower by ~2‰ in the PEPC-OE compared to WT plants, across the pCO2 experimental range. Although in PEPC-OE plants there was a tendency for ∆bio to decrease with lower pCO2 (i.e., β to increase, in accordance with Abadie and Tcherkez 2018), the CO2 dependency was likely minimized due to the low photorespiratory measurement conditions (Leegood and von Caemmerer 1988, 1989, 1994). However, across the pCO2 experimental interval the calculated mean β was ~6% in PEPC-OE plants compared to zero in WT (Fig. 2b). The significant change in ∆bio and increase in β indicate a contribution of ZmPEPC to carboxylation in the PEPC-OE plants compared to WT even though there was no detectable difference in A between the plant types. This might suggest either that carbon carboxylated by PEPC did not go through Calvin cycle (Häusler et al. 1999) or that Rubisco had a lower carboxylation efficiency in the PEPC-OE plants compared to WT (Agarie et al. 2002; Fukayama et al. 2003). The latter explanation seems unlikely in the present study, where slightly higher Rubisco activity was determined in the PEPC-OE plants compared to WT, and comparable maximum carboxylation efficiency values (µmol CO2 m−2 s−1 Pa−1) were calculated for the two plant types (data not shown). Alternatively, based on the collective information from previous studies, overexpression of PEPC may have enhanced the anaplerotic pathway (Fukayama et al. 2003; Miyao and Fukayama 2003; O’Leary et al. 2011; Kandoi et al. 2016; Abadie and Tcherkez 2018) rather than promoting carbon fixation of the C4-like photosynthetic pathway.
The difference in b between the PEPC-OE and WT plants could in part be affected by the magnitude of \(\frac{{R_{\text{L}} e^{*} }}{{\left( {A + R_{\text{L}} } \right)}}\) (that contributes to ∆bio_mod in Eq. 1) in the transgenic plants. However, in the current study, the RL/(A + RL) ratio was low at all three Ca. In addition, given the relatively low sensitivity of b to RL (see Fig. S4A), even a higher RL in the PEPC-OE in comparison to WT plants will have a minor contribution to the 13C discrimination analysis.
In the present study, leaf photosynthetic measurements were conducted under low pO2 (1.84 kPa) to reduce the uncertain contributions of photorespiration and potential re-fixation of (photo)respired CO2 by PEPC and Rubisco (Ku et al. 2000; Agarie et al. 2001) to leaf net discrimination against 13CO2, and therefore to minimize the errors in ∆bio estimate. In addition, since low pO2 reduces the inhibition of RL relative to rates of leaf respiration in the dark (Rd) (Abadie et al. 2017; Tcherkez et al. 2017a, b; Gauthier et al. 2018), RL was modelled at 25 °C from Rd at 30 °C after three hours from the light–dark transition. Based on previous studies, there is indication of negative effects exerted by the atmospheric CO2 level, in the short-term, on leaf respiration activity in the light (Tcherkez et al. 2008, 2017a). Since the predicted RL were applied over all measurement CO2 conditions, a potential overestimation of RL may have therefore risen at the highest Ca; nevertheless, its effect on the 13C discrimination analysis is considered minor given the low RL/(A + RL) ratio. Since there is no evidence in the literature of different downregulation of RL in transgenic PEPC-OE compared WT rice the higher RL predicted for the PEPC-OE compared to WT plants may be due to ZmPEPC activity.
In the ∆bio analysis, the gm values determined on WT based on leaf net discrimination against 13CO2 were applied to PEPC-OE plants. This assumption of equal gm in transgenic and WT plants was supported by nonsignificantly different gm determined by leaf 18O discrimination (Yakir 1998; Gillon and Yakir 2000; Barbour et al. 2016; Ubierna et al. 2017) on the PEPC-OE and WT plants under the same experimental conditions of the present study (Table S2). It is theoretically possible that the increased PEPC activity in the transgenic plants would enhance gm compared to WT, as previously reported by Alonso-Cantabrana and von Caemmerer (2016) for a C3–C4 intermediate species. However, an increase in gm would lead to a further decrease in the estimate of ∆bio as a 0.5 µmol CO2 m−2 s−1 Pa−1 raise in gm lowers ∆bio by ~1‰ (Fig. S3).
Furthermore, in the present study the ∆bio analysis assumed no 13CO2 respiratory fractionation via TCA cycle (e, ‰; Ghashghaie et al. 2003; Werner and Gessler 2011); however, even a large e would have exerted a minor effect on b and gm, as presented in Method S5 Fig. S4C and S4D, respectively.
Leaf dark respiration and 13C composition of evolved CO2 in PEPC-OE versus WT plants
In the present study, following leaf transition from light to dark, Rd, and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) showed a hyperbolic decrease over a three-hour period in both plant types, with a significantly higher Rd and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) in the PEPC-OE compared to WT plants (Table S4 and Table S5). It has been previously shown that the high Rd in the first 30 min after light–dark transition, light enhanced dark respiration (LEDR), results primarily from leaf respiration of substrates as organic acids (in particular malate), produced in the prior light period (Werner and Gessler 2011; Tcherkez et al. 2012, 2015, 2016b; Gessler et al. 2017). Based on the analysis conducted on several species, Lehman et al. (2016b) reported how the leaf respiration rate and 13C composition of evolved CO2 during the LEDR time may be only weakly related, and how changes in both responses are highly species-specific. In the present study, for both plant types, a close correlation between Rd and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) over the three-hour dark period was determined (r > 0.90). However, leaf CO2 evolution in the dark and its \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) composition contain information about leaf metabolism and respiratory substrates (Lehman et al. 2016b). For example, Barbour et al. (2007) and Gessler et al. (2009) reported for castor bean (Ricinus communis) that leaf LEDR mainly comes from the decarboxylation of 13C heavier metabolites, mostly malate, and that the declines in LEDR and \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) over time are caused by the decrease in malate availability as respiratory substrate. In the dark, leaf malate can be decarboxylated via malic enzyme in mitochondria to make pyruvate, or alternatively, it can be oxidized to OAA via NAD-MDH (Wiskich and Dry 1985; Douce and Neuburger 1987). Pyruvate and OAA can subsequently be used in anaplerotic reactions to replenish TCA cycle intermediates when they are consumed for lipid or amino acid synthesis (Doubnerová and Ryšlavá 2011; Muramatsu et al. 2014; Lehmann et al. 2015, 2016b). In the current study, the Rd integral over 30 min after light–dark transition was 0.48 mmol CO2 m−2 higher in PEPC-OE with respect to WT plants. Theoretically, if the enhancement of LEDR in the PEPC-OE plants during the first 30 min in the dark was due to malate alone, this would have required total 0.12 mmol malate m−2, i.e., ~ the double of the leaf malate content determined during the photoperiod in PEPC-OE plants. Since no significant difference in leaf malate accumulation was observed between PEPC-OE and WT plants, other leaf organic acids (e.g., fumarate, citrate; Agarie et al. 2002; Abadie and Tcherkez 2018) produced in the light duration may have also contributed to a bigger pool of dark respiratory substrates in the PEPC-OE compared to WT (Tcherkez et al. 2012; Lehman et al. 2016b). In addition, part of the leaf malate may be in an inactive pool (e.g., in the vacuole) as observed in C4 plants (Hatch 1979; Arrivault et al. 2017) and therefore not readily available for LEDR. Another carbon source for Rd in the PEPC-OE plants could be via function of PEPC in the dark, when ZmPEPC has been reported to be in a phosphorylated status (Fukayama et al. 2003; Leegood 2013). In the dark period, by utilizing part of the PEP produced during glycolysis, ZmPEPC in rice could lead to the increased synthesis of malate that can be metabolized in the TCA cycle as substrate for anaplerosis during mitochondrial respiration, with the possibility to raise the CO2 evolution (Suzuki et al. 2006).
Over the 3 h in the dark, the variations in \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) for both PEPC-OE and WT plants suggest a decrease in the contribution to Rd of respiratory substrates produced in the leaf chamber (Lch) before the light–dark transition and a complementary increase in the contribution of substrates produced in the growth chamber. The Lch carbon assimilates were estimated to account from ~50% of the substrates for Rd after 6 min to ~30% after 30 min, and only ~10% after three hours in the dark, with no differences between transgenic and WT plants (see Fig. S5). Tcherkez et al. (2010) estimated that recent carbon assimilates in sunflower (Helianthus annuus) provided 40–60% of substrates for leaf respiration (via a pool with a half-life of several hours) both in the light and in the dark; similar contribution was determined by Nogués et al. (2004) on French bean (Phaseolus vulgaris) for approximately two-hour dark after leaf illumination. The higher \(\delta^{ 1 3} {\text{C}}_{\text{Rd}}\) responses in PEPC-OE plants compared to WT during LEDR may partially depend on a relatively greater contribution to leaf dark respiration of organic acids, which are 13C enriched, compared to other respiratory substrates as sugars and amino acids (Lehman et al. 2016b). In particular, during the photoperiod, the PEPC activity in the PEPC-OE plants could promote the production of 13C-enriched OAA, compared to WT, which can be converted to malate by MDH and used to feed leaf respiration after light–dark transition (Barbour et al. 2007; Gessler et al. 2009; Werner et al. 2011; Lehmann et al. 2016b). In addition, the tendency of a higher \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}( 2 4 {\text{h}})}} \,\left( {{\text{and}}\,\delta^{ 1 3} {\text{C}}_{\text{dm}} } \right)\) in PEPC-OE versus WT plants may indicate that the substrates available for the TCA cycle produced in the Gch had a slightly more enriched 13C composition in the transgenic plants compared to WT. This may suggest that in the growth chamber at current atmospheric pO2 and pCO2 of 184 Pa, a possible lower leaf net discrimination against 13CO2 could have occurred in the PEPC-OE plants compared to WT. For both plant types, a lower \(\delta^{ 1 3} {\text{C}}_{{{\text{Rd}}( 2 4 {\text{h}})}}\) than \(\delta^{ 1 3} {\text{C}}_{\text{dm}}\) is in agreement with Tcherkez et al. (2003).
Conclusions
There are uncertainties in the physiological effect of transgenic expression of ZmPEPC in the C3 plant rice. However, enhancement of PEPC activity is a key step in engineering C4 photosynthesis into C3 plants. In the present study, the transgenic rice plants expressing ZmPEPC had higher in vitro PEPC activity, a significant fraction of carbon fixed by PEPC and a decreased ∆bio compared to WT (determined at pCO2 from below to above current ambient level). However, A was not significantly different between PEPC-OE and WT plants, while Rd and 13C composition of leaf dark-evolved CO2 were higher in the PEPC-OE plants versus WT, additionally indicating enhanced in vivo PEPC activity in the PEPC-OE plants. These results suggest that although ZmPEPC appears to be functional in the PEPC-OE rice plants, there are some factors likely related to substrate availability (PEP and/or bicarbonate) or posttranslational controls (e.g., involving regulatory phosphorylation) that reduce the activity of the enzyme in vivo during the photoperiod. Insights into these limitations may be discernible with detailed analysis of metabolite pools (organic acids, carbohydrates, and starch), ad hoc estimates of gm, and magnitude of refixation of photorespired CO2 compared to WT. This will provide the much needed understanding to further the development of a functioning C4 photosynthetic cycle in rice.
References
Abadie C, Tcherkez G (2018) In vivo phosphoenolpyruvate carboxylase activity is controlled by CO2 and O2 mole fractions and represents a major flux at high photorespiration rates. New Phytol 218:94–106
Abadie C, Blanchet S, Carrol A, Tcherkez G (2017) Metabolomics analysis of post-photosynthetic effects of gaseous O2 on primary metabolism of illuminated leaves. Funct Plant Biol 44:929–940
Agarie S, Sasaki H, Matsuoka M, Miyao-Tokutomi M (2001) Overexpression of C4 phosphoenolpyruvate carboxylase increased carbon isotope discrimination in transgenic rice plants. Plant Prod Sci 4:311–312
Agarie S, Miura A, Sumikura R, Sukamoto ST, Nose A, Arima S, Matsuoka M, Miyao-Tokutomi M (2002) Overexpression of C4 PEPC caused O2-insensitive photosynthesis in transgenic rice plants. Plant Sci 162:257–265
Alonso-Cantabrana H, von Caemmerer S (2016) Carbon isotope discrimination as a diagnostic tool for C4 photosynthesis in C3–C4 intermediate species. J Exp Bot 67:3109–3121
Arrivault S, Obata T, Szecówka M, Mengin V, Guenther M, Hoehne M, Fernie RA, Stitt M (2017) Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. J Exp Bot 68:283–298
Atkin OK, Evans JR, Siebke K (1998) Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Funct Plant Biol 25:437–443
Barbour MM, McDowell NG, Tcherkez G, Bickford CP, Hanson DT (2007) A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ 30:469–482
Barbour MM, Warren CR, Farquhar GD, Forrester GUY, Brown H (2010) Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant Cell Environ 33:1176–1185
Barbour MM, Evans JR, Simonin KA, Caemmerer S (2016) Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytol 210:875–889
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259
Bickford CP, Hanson DT, McDowell NG (2010) Influence of diurnal variation in mesophyll conductance on modelled 13C discrimination: results from a field study. J Exp Bot 61:3223–3233
Brugnoli E, Hubick KT, von Caemmerer S, Wong SC, Farquhar GD (1988) Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol 88:1418–1424
Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD (2013) Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol 200:950–965
Cousins AB, Baroli I, Badger MR, Ivakov A, Lea PJ, Leegood RC, von Caemmerer S (2007) The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol 145:1006–1017
Doubnerová V, Ryšlavá H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180:575–583
Douce R, Neuburger M (1987) General organization of the respiratory chain and matrix-associated specific dehydrogenases in higher plant mitochondria. In: Moore A, Beechey R (eds) Plant mitochondria: structural, functional, and physiological aspects. Plenum Press, New York, pp 1–15
Edwards GE, Ku MSB, Hatch MD (1982) Photosynthesis in Panicum milioides, a species with reduced photorespiration. Plant Cell Physiol 23:1185–1195
Evans JR, von Caemmerer S (2013) Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ 36:745–756
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Funct Plant Biol 13:281–292
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, Ehleringer J, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Fukayama H, Hatch MD, Tamai T, Tsuchida H, Sudoh S, Furbank RT, Miyao M (2003) Activity regulation and physiological impacts of maize C4-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants. Photosynth Res 77:227–239
Gauthier PP, Battle MO, Griffin KL, Bender ML (2018) Measurement of gross photosynthesis, respiration in the light, and mesophyll conductance using H 182 O labeling. Plant Physiol 177:62–74
Gehlen J, Panstruga R, Smets H et al (1996) Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol Biol 32:831–848
Gessler A, Tcherkez G, Karyanto O, Keitel C, Ferrio JP, Ghashghaie J, Kreuzwieser J, Farquhar GD (2009) On the metabolic origin of the carbon isotope composition of CO2 evolved from darkened light-acclimated leaves in Ricinus communis. New Phytol 181:374–386
Gessler A, Roy J, Kayler Z, Ferrio JP et al (2017) Night and day—Circadian regulation of night-time dark respiration and light-enhanced dark respiration in plant leaves and canopies. Environ Exp Bot 137:14–25
Ghashghaie J, Badeck F-W, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2:145–161
Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20:1217–1230
Gillon JS, Yakir D (2000) Internal conductance to CO2 diffusion and C18OO discrimination in C3 Leaves. Plant Physiol 123:201–214
Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE (2013) Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (Genus Oryza). Plant Physiol 162:1632–1651
Giuliani R, Karki S, Covshoff S, et al (2019) Transgenic maize phosphoenolpyruvate carboxylase alters leaf–atmosphere CO2 and 13CO2 exchange in Oryza sativa. J Exp Bot 70:2773–2786
Gong XY, Schäufele R, Feneis W, Schnyder H (2015) 13CO2/12CO2 exchange fluxes in a clamp-on leaf cuvette: disentangling artefacts and flux components. Plant Cell Environ 38:2417–2432
Griffiths H, Cousins AB, Badger MR, von Caemmerer S (2007) Discrimination in the dark. Resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the crassulacean acid metabolism cycle. Plant Physiol 143:1055–1067
Hatch MD (1979) Mechanism of C4 photosynthesis in Chloris gayana: pool sizes and kinetics of 14CO2 incorporation into 4-carbon and 3-carbon intermediates. Arch Biochem Biophys 194:117–127
Häusler RE, Kleines M, Uhrig H, Hirsch H-J, Smets H (1999) Overexpression of phosphoenolpyruvate carboxylase from Corynebacterium glutamicum lowers the CO2 compensation point (Γ*) and enhances dark and light respiration in transgenic potato. J Exp Bot 50:1231–1242
Häusler RE, Hirsch HJ, Kreuzaler F, Peterhansel C (2002) Overexpression of C4-cycle enzymes in transgenic C3 plants: a biotechnological approach to improve C3-photosynthesis. J Exp Bot 53:591–607
Hiei Y, Komari T (2006) Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 85:271–283
Hu L, Li Y, Xu W, Zhang Q, Zhang L, Qi X, Dong H (2012) Improvement of the photosynthetic characteristics of transgenic wheat plants by transformation with the maize C4 phosphoenolpyruvate carboxylase gene. Plant Breeding 131:385–391
Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MSB (1992) Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco. Plant Physiol 98:458–464
Ivlev AA, Bykova NV, Igamberdiev AU (1996) Fractionation of carbon (13C/12C) isotopes in glycine decarboxylase reaction. FEBS Lett 386:174–176
Jeanneau M, Vidal J, Gousset-Dupont A, Lebouteiller B, Hodges M, Gerentes D, Perez P (2002) Manipulating PEPC levels in plants. J Exp Bot 53:1837–1845
Jiao D, Huang X, Li X, Chi W, Kuang T, Zhang Q, Ku MSB, Cho D (2002) Photosynthetic characteristics and tolerance to photo-oxidation of transgenic rice expressing C4 photosynthesis enzymes. Photosynth Res 72:85–93
Kandoi D, Mohanty S, Tripathy BC (2016) Towards efficient photosynthesis: overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth Res 130:1–26
Koteyeva NK, Voznesenskaya EV, Edwards GE (2015) An assessment of the capacity for phosphoenolpyruvate carboxykinase to contribute to C4 photosynthesis. Plant Sci 235:70–80
Ku MSB, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M (1999) High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17:76–80
Ku MSB, Cho D, Ranade U, Hsu TP, Li X, Jiao DM, Ehleringer JM, Miyao M, Matsuoka M (2000) Photosynthetic performance of transgenic rice plants overexpressing maize C4 photosynthesis enzymes. In: Sheehy JE, Mitchell PL, Hardy B (eds) Redesigning rice photosynthesis to increase yield. Elsevier Science/IRRI, Amsterdam, pp 193–204
Lanigan GJ, Betson N, Griffiths H, Seibt U (2008) Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiol 148:2013–2020
Le Roux X, Bariac T, Sinoquet H, Genty B, Piel C, Mariotti A, Girardin C, Richard P (2001) Spatial distribution of leaf water-use efficiency and carbon isotope discrimination within an isolated tree crown. Plant Cell Environ 24:1021–1032
Leegood RC (2013) Strategies for engineering C4 photosynthesis. J Plant Physiol 170:378–388
Leegood RC, von Caemmerer S (1988) The relationship between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Amaranthus edulis. Planta 174:253–262
Leegood RC, von Caemmerer S (1989) Some relationships between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Zea mays L. Planta 178:258–266
Leegood RC, von Caemmerer S (1994) Regulation of photosynthetic carbon assimilation in leaves of C3–C4 intermediate species of Moricandia and Flaveria. Planta 192:232–238
Lehmann MM, Rinne KT, Blessing C, Siegwolf RTW, Buchmann N, Werner RA (2015) Malate as a key carbon source of leaf dark-respired CO2 across different environmental conditions in potato plants. J Exp Bot 66:5769–5781
Lehmann MM, Wegener F, Barthel M, Maurino VG, Siegwolf RT, Buchmann N, Werner C, Werner RA (2016a) Metabolic fate of the carboxyl groups of malate and pyruvate and their influence on δ 13C of leaf-respired CO2 during light enhanced dark respiration. Front Plant Sci 7:739
Lehmann MM, Wegener F, Werner RA, Werner C (2016b) Diel variations in carbon isotopic composition and concentration of organic acids and their impact on plant dark respiration in different species. Plant Biol 18:776–784
Lian L, Wang X, Zhu Y, He W, Cai Q, Xie H, Zhang M, Zhang J (2014) Physiological and photosynthetic characteristics of indica Hang2 expressing the sugarcane PEPC gene. Mol Biol Rep 41:2189–2197
Lin HC, Karki S, Coe RA et al (2016) Targeted knockdown of GDCH in rice leads to a photorespiratory deficient phenotype useful as a building block for C4 rice. Plant Cell Physiol 57:919–932
Matsuoka M, Furbank RT, Fukayama H, Miyao M (2001) Molecular engineering of C4 photosynthesis. Annu Rev Plant Biol 52:297–314
Miyao M (2003) Molecular evolution and genetic engineering of C4 photosynthetic enzymes. J Exp Bot 54:179–189
Miyao M, Fukayama H (2003) Metabolic consequences of overproduction of phosphoenolpyruvate carboxylase in C3 plants. Arch Biochem Biophys 414:197–203
Muramatsu M, Suzuki R, Yamazaki T, Miyao M (2014) Comparison of plant-type phosphoenolpyruvate carboxylases from rice: identification of two plant-specific regulatory regions of the allosteric enzyme. Plant Cell Physiol 56:468–480
Nogués S, Tcherkez G, Cornic G, Ghashghaie J (2004) Respiratory carbon metabolism following illumination in intact French bean leaves using 12C/13C labeling. Plant Physiol 136:3245–3254
O’Leary B, Park J, Plaxton WC (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436:15–34
Qi X, Xu W, Zhang J, Guo R, Zhao M, Hu L, Wang H, Dong H, Li Y (2017) Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 254:1017–1030
Rademacher T, Häusler RE, Hirsch H-J, Zhang L, Lipka V, Weier D, Kreuzaler F, Peterhänsel C (2002) An engineered phosphoenolpyruvate carboxylase redirects carbon and nitrogen flow in transgenic potato plants. Plant J 32:25–39
Roeske CA, O’Leary MH (1984) Carbon isotope effects on enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23:6275–6284
Rooney MA (1988) Short-term carbon isotopic fractionation in plants. PhD thesis, Wisconsin University
Sharkey TD, Berry JA, Raschke K (1985) Starch and sucrose synthesis in Phaseolus vulgaris as affected by light, CO2, and abscisic acid. Plant Physiol 77:617–620
Sonawane BV, Cousins AB (2018) Uncertainties and limitations of using carbon-13 and oxygen-18 leaf isotope exchange to estimate the temperature response of mesophyll CO2 conductance in C3 plants. New Phytol 222:122–131
Stutz SS, Edwards GE, Cousins AB (2014) Single-cell C4 photosynthesis: efficiency and acclimation of Bienertia sinuspersici to growth under low light. New Phytol 202:220–232
Sun W, Ubierna N, Ma J-Y, Walker BJ, Kramer DM, Cousins AB (2014) The coordination of C4 photosynthesis and the CO2-concentrating mechanism in maize and Miscanthus x giganteus in response to transient changes in light quality. Plant Physiol 164:1283–1292
Suzuki S, Murai N, Kasaoka K, Hiyoshi T, Imaseki H, Burnell JN, Arai M (2006) Carbon metabolism in transgenic rice plants that express phosphoenolpyruvate carboxylase and/or phosphoenolpyruvate carboxykinase. Plant Sci 170:1010–1019
Taniguchi Y, Ohkawa H, Masumoto C et al (2008) Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J Exp Bot 59:1799–1809
Tazoe Y, von Caemmerer S, Badger MR, Evans JR (2009) Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. J Exp Bot 60:2291–2301
Tazoe Y, von Caemmerer S, Estavillo GM, Evans JR (2011) Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant Cell Environ 34:580–591
Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol 131:237–244
Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J (2005) In Vivo respiratory metabolism of illuminated leaves. Plant Physiol 138:1596–1606
Tcherkez G, Bligny R, Gout E, Mahé A, Hodges M, Cornic G (2008) Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proc Natl Acad Sci USA 105:797–802
Tcherkez G, Schaufele R, Nogues S et al (2010) On the 13C/12C isotopic signal of day and night respiration at the mesocosm level. Plant Cell Environ 33:900–913
Tcherkez G, Boex-Fontvieille E, Mahé A, Hodges M (2012) Respiratory carbon fluxes in leaves. Curr Opin Plant Biol 5:308–314
Tcherkez G, Gauthier P, Buckley TN et al (2017a) Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. Tansley review. New Phytol 216:986–1001
Tcherkez G, Gauthier P, Buckley TN et al (2017b) Tracking the origins of the Kok effect, 70 years after its discovery. New Phytol 214:506–510
Ubierna N, Farquhar GD (2014) Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant Cell Environ 37:1494–1498
Ubierna N, Sun W, Kramer DM, Cousins AB (2013) The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus x giganteus and Flaveria bidentis. Plant Cell Environ 36:365–381
Ubierna N, Gandin A, Boyd RA, Cousins AB (2017) Temperature response of mesophyll conductance in three C4 species calculated with two methods: 18O discrimination and in vitro Vpmax. New Phytol 214:66–80
Vidal J, Chollet R (1997) Regulatory phosphorylation of C4 PEP carboxylase. Trends Plant Sci 2:230–237
von Caemmerer S (1989) A model of photosynthetic CO2 assimilation and carbon-isotope discrimination in leaves of certain C3–C4 intermediates. Planta 178:463–474
von Caemmerer S, Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplasts from leaves of severa1 C3 plants. Aust J Plant Physiol 18:287–305
von Caemmerer S, Ghannoum O, Pengelly JJL, Cousins AB (2014) Carbon isotope discrimination as a tool to explore C4 photosynthesis. J Exp Bot 65:3459–3470
Walker B, Ariza LS, Kaines S, Badger MR, Cousins AB (2013) Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ 36:2108–2119
Weber AP, von Caemmerer S (2010) Plastid transport and metabolism of C3 and C4 plants—comparative analysis and possible biotechnological exploitation. Curr Opin Plant Biol 13:257–265
Werner C, Gessler A (2011) Diel variations in the carbon isotope composition of respired CO2 and associated carbon sources: a review of dynamics and mechanisms. Biogeosciences 8:2437–2459
Werner RA, Buchmann N, Siegwolf RTW, Kornexl BE, Gessler A (2011) Metabolic fluxes, carbon isotope fractionation and respiration—lessons to be learned from plant biochemistry. New Phytol 191:10–15
Wingate L, Seibt U, Montcrieff JB, Jarvis PG, Lloyd JOL (2007) Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant Cell Environ 30:600–616
Wiskich J, Dry I (1985) The tricarboxylic acid cycle in plant mitochondria: its operation and regulation. In: Douce R, Day D (eds) Higher Plant Cell Respiration. Springer, New York, pp 281–313
Yakir D (1998) Oxygen-18 of leaf water: a crossroad for plant-associated isotopic signals. In: Griffiths H (ed) Stable isotopes: integration of biological, ecological and geochemical processes. BIOS Scientific Publishers, Oxford, pp 147–168
Yoshida S, Forno DA, Cock JH, Gomez KA (1972) Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Baños
Zhu XG, Shan L, Wang Y, Quick WP (2010) C4 rice—an ideal arena for systems biology research. J Integr Plant Biol 52:762–770
Acknowledgements
This research was funded by the C4 Rice Project grant from The Bill and Melinda Gates Foundation to IRRI (2012–2015) and to the University of Oxford (2015–2019); by the National Science Foundation, Grant MCB-1146928; by the National Science Foundation, Grant MRI-0923562; and by the Russian Science Foundation, Grant 16-16-00089. We thank Charles A. Cody for plant growth management, Allison R. Kolbe for assistance determining PEPC activity, and Raymond W. Lee for 13C analysis on leaf biomass.
Author information
Authors and Affiliations
Contributions
SK, SC, H-CL, RAC, WPQ, and JMH generated the transgenic plant material; SvC, RG, RTF, GEE, and ABC planned and designed the experiments; RG performed leaf–atmosphere gas and isotope exchange measurements and analyses, and NK performed the biochemical analysis; RG, SvC, RTF, GEE, and ABC interpreted the data; and RG, ABC, and GEE developed and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Giuliani, R., Karki, S., Covshoff, S. et al. Transgenic maize phosphoenolpyruvate carboxylase alters leaf–atmosphere CO2 and 13CO2 exchanges in Oryza sativa. Photosynth Res 142, 153–167 (2019). https://doi.org/10.1007/s11120-019-00655-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00655-4