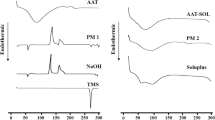
Abstract
Purpose
While including amorphous solid dispersion (ASD) in tablet formulations is increasingly common, tablets containing high ASD loading are associated with slow disintegration, which presents a challenge to control pill burden for less potent compounds.
Methods
We use a model ASD, composed of a hydrophobic drug with copovidone and a non-ionic surfactant, to explore formulation options that can prevent slow disintegration.
Results
In addition to the ASD loading, the pH of the disintegration medium and the inclusion of inorganic salts in the tablet also have an impact on the tablet disintegration time. Certain kosmotropic salts, when added in the formulation, can significantly accelerate tablet disintegration, though the rank order in their effectiveness does not exactly follow the Hofmeister series at pH 1.8. The particle size and dissolution rate of the salt can contribute to its overall effectiveness.
Conclusion
We provided a mechanistic explanation of the disintegration process: fast-dissolving kosmotropic salt results in a concentrated salt solution inside the restrained tablet matrix, thus inhibiting the dissolution of copovidone and preventing polymer gelling which is the main cause leading the slow disintegration. The outcome of this study has enabled the design of a higher ASD loading platform formulation for copovidone based ASD.

MicroCT aids the mechanistic understanding of the role of inorganic salt in the tablet disintegration of amorphous solid dispersion based formulation






Similar content being viewed by others
References
Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci. 1971;60(9):1281–302.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliver Rev. 2001;48(1):27–42.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60.
DiNunzio JC, Schilling SU, Coney AW, Hughey JR, Kaneko N, McGinity JW. Use of highly compressible Ceolus (TM) microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev Ind Pharm. 2012;38(2):180–9.
Goddeeris C, Willems T, Van den Mooter G. Formulation of fast disintegrating tablets of ternary solid dispersions consisting of TPGS 1000 and HPMC 2910 or PVPVA 64 to improve the dissolution of the anti-HIV drug UC 781. Eur J Pharm Sci. 2008;34(4–5):293–302.
Hughey JR, Keen JM, Miller DA, Kolter K, Langley N, McGinity JW. The use of inorganic salts to improve the dissolution characteristics of tablets containing Soluplus (R)-based solid dispersions. Eur J Pharm Sci. 2013;48(4–5):758–66.
Sarkar N. Thermal gelation properties of methyl and Hydroxypropyl methylcellulose. J Appl Polym Sci. 1979;24(4):1073–87.
Joshi SC. Sol-gel behavior of Hydroxypropyl methylcellulose (HPMC) in ionic media including drug release. Materials. 2011;4(10):1861–905.
Aseyev V, Tenhu H, Winnik FM. Non-ionic Thermoresponsive polymers in water. Adv Polym Sci. 2011;242:29–89.
Florin E, Kjellander R, Eriksson JC. Salt effects on the cloud point of the poly (ethylene oxide) + water-system. J Chem Soc Farad T 1. 1984;80:2889–2910.
Touitou E, Donbrow M. Influence of additives on (Hydroxyethyl) methylcellulose properties - relation between gelation temperature-change, compressed matrix integrity and drug release profile. Int J Pharm. 1982;11(2):131–48.
Zhang YJ, Furyk S, Bergbreiter DE, Cremer PS. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J Am Chem Soc. 2005;127(41):14505–10.
Alexandridis P, Holzwarth JF. Differential scanning calorimetry investigation of the effect of salts on aqueous solution properties of an amphiphilic block copolymer (Poloxamer). Langmuir. 1997;13(23):6074–82.
Collins KD. Charge density-dependent strength of hydration and biological structure. Biophys J. 1997;72(1):65–76.
Hribar B, Southall NT, Vlachy V, Dill KA. How ions affect the structure of water. J Am Chem Soc. 2002;124(41):12302–11.
Omta AW, Kropman MF, Woutersen S, Bakker HJ. Negligible effect of ions on the hydrogen-bond structure in liquid water. Science. 2003;301(5631):347–9.
Yang YY, Zeng F, Tong Z, Liu XX, Wu SZ. Phase separation in poly(N-isopropyl acrylamide)/water solutions. II. Salt effects on cloud-point curves and gelation. J Polym Sci Pol Phys. 2001;39(9):901–7.
Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996;71(4):2056–63.
Hofmeister F. Zur Lehre von der wirkung der saltze. Naunyn Schmiedeberg's Arch Pharmacol. 1888;24:247.
Zhang Y, Furyk S, Sagle LB, Cho Y, Bergbreiter DE, Cremer PS. Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J Phys Chem C. 2007;111(25):8916–24.
Pygall SR, Kujawinski S, Timmins P, Melia CD. Mechanisms of drug release in citrate buffered HPMC matrices. Int J Pharm. 2009;370(1–2):110–20.
Fagan PG, Harrison PJ, Shankland N. A correlation between cloud point and disintegration of hydroxyalkylcellulose controlled release matrices. J Pharm Pharmcol. 1989;41:25.
Mitchell K, Ford JL, Armstrong DJ, Elliott PNC, Rostron C, Hogan JE. The influence of additives on the cloud point, disintegration and dissolution of Hydroxypropylmethylcellulose gels and matrix tablets. Int J Pharm. 1990;66(1–3):233–42.
Liu SQ, Joshi SC, Lam YC. Effects of salts in the Hofmeister series and solvent isotopes on the gelation mechanisms for hydroxypropylmethylcellulose hydrogels. J Appl Polym Sci. 2008;109(1):363–72.
Kajiyama A, Takagi H, Moribe K, Yamamoto K. Improvement of HPMC tablet disintegration by the addition of inorganic salts. Chem Pharm Bull. 2008;56(4):598–601.
Scott B, Zhang K, Wigman L. Material identification by HPLC with charged aerosol detection. Lc Gc N Am. 2013:37–41.
Bolhuis GK, Zuurman K, teWierik GHP. Improvement of dissolution of poorly soluble drugs by solid deposition on a super disintegrant .2. The choice of super disintegrants and effect of granulation. Eur J Pharm Sci 1997;5(2):63–69.
Zhao N, Augsburger LL. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. Aaps Pharmscitech. 2005;6(1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 375 kb)
Rights and permissions
About this article
Cite this article
Xi, H., Ren, J., Novak, J.M. et al. The Effect of Inorganic Salt on Disintegration of Tablets with High Loading of Amorphous Solid Dispersion Containing Copovidone. Pharm Res 37, 70 (2020). https://doi.org/10.1007/s11095-020-2772-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-2772-7