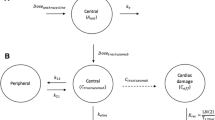
ABSTRACT
Purpose
Trastuzumab treatment is associated with occurrence of cardiac toxicity, for which monitoring of the left ventricular ejection fraction (LVEF) is indicated. The performance of the currently used monitoring protocol as defined in the summary of product characteristics (SPC) is however unknown. The objective of this analysis was to develop a model-based framework for evaluation and optimization of cardiac monitoring strategies.
Methods
The model-based framework comprised a previously developed exposure-response model for trastuzumab induced changes in LVEF, and a protocol-execution model that allowed incorporation of treatment interventions as described by a monitoring protocol. Metrics for evaluation of toxicity, dose intensity and monitoring burden were defined to allow evaluation and optimization of cardiac monitoring protocols.
Results
The success of a protocol-defined dose reduction was improved from 40% for the SPC-based protocol, to 79% for a scoring-based protocol, thereby decreasing the observed severity of cardiotoxicity. Including adaptation based on risk-profile allowed reduction of the mean number of LVEF measurements by 19%.
Conclusions
This model-based evaluation approach enabled evaluation and optimization of cardiac monitoring protocols that would be difficult to evaluate in a clinical setting. This approach can potentially be applied for other drugs that use repeated evaluation of continuous biomarkers for toxicity.






Similar content being viewed by others
Abbreviations
- AUC45 :
-
area below a LVEF threshold of 45% and above the LVEF time curve
- FT-AUC45 :
-
full-treatment AUC45
- LVEF:
-
left ventricular ejection fraction
- O-AUC45 :
-
observed AUC45
- PD:
-
pharmacodynamics
- PK:
-
pharmacokinetics
- SPC:
-
summary of product characteristics
REFERENCES
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–84.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Smith I, Procter M, Gelber R, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36.
Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2007;25(25):3859–65.
Tan-Chiu E, Yothers G, Romond E, Geyer Jr CE, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast can. J Clin Oncol. 2005;23:7811–9.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65.
Fuchs IB, Landt S, Bueler H, Kuehl U, Coupland S, Kleine-Tebbe A, et al. Analysis of HER2 and HER4 in human myocardium to clarify the cardiotoxicity of trastuzumab (Herceptin). Breast Cancer Res Treat. 2003;82:23–8.
Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the herceptin adjuvant (HERA) trial. J Clin Oncol. 2010;28(21):3422–8.
Russell SD, Blackwell KL, Lawrence J, Pippen JE, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the Use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the national surgical adjuvant breast and bowel project B-31 and the No. J Clin Oncol. 2010;28(21):3416–21.
Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–6.
van Hasselt JGC, Boekhout AH, Beijnen JH, Schellens JHM, Huitema ADR. Population pharmacokinetic-pharmacodynamic analysis of trastuzumab-associated cardiotoxicity. Clin Pharmacol Ther. 2011;90(1):126–32.
Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25(25):3808–15.
Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Annals of oncology. 2012 Jun 13
Summary of Product Characteristics Trastuzumab [Internet]. [cited 2011 Oct 27];Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf
R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2011.
Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: package Desolve. J Stat Softw. 2010;33(9):1–25.
Buxton A, Ellison K, Lorvidhaya P, Ziv O. Left ventricular ejection fraction for sudden death risk stratification and guiding implantable cardioverter-defibrillators implantation. J Cardiovasc Pharmacol. 2010;55(5):450–5.
Subar M, Lin W, Chen W, Pittman DG. Lack of uniformity in cardiac assessment during trastuzumab therapy. The Breast Journal. 2011;17(4):383–90.
Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S, et al. Rationale and design of the multidisciplinary approach to novel therapies in cardiology oncology research trial (MANTICORE 101–Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzu. BMC cancer. 2011;11:318.
Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol: Official Journal of the American Society of Clinical Oncology. 2010;28(21):3429–36.
Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Hear J. 1998;135(5 Pt 1):825–32.
Broeyer FJF, Osanto S, van Eck HJ R, van Steijn AQMJ, Ballieux BEPB, Schoemaker RC, et al. Evaluation of biomarkers for cardiotoxicity of anthracyclin-based chemotherapy. J Cancer Res Clin Oncol. 2008;134(9):961–8.
ACKNOWLEDGMENTS and Disclosures
No funding was received for the conduct of this project. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Hasselt, J.G.C., Schellens, J.H.M., Mac Gillavry, M.R. et al. Model-Based Evaluation and Optimization of Cardiac Monitoring Protocols for Adjuvant Treatment of Breast Cancer with Trastuzumab. Pharm Res 29, 3499–3511 (2012). https://doi.org/10.1007/s11095-012-0845-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0845-y