Abstract
Purpose
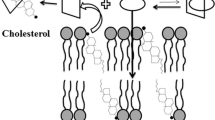
This study examines the effect of a chemically modified β-cyclodextrin on the liposome bilayer permeability of a liposomally entrapped model hydrophobic weak acid, DB-67 (7-t-butyldimethylsilyl-10-hydroxycamptothecin).
Materials and Methods
Permeability studies were conducted in liposomes prepared by hydration–extrusion in the presence or absence of entrapped hydroxypropyl-β-cyclodextrin (HPβCD). A gradient HPLC method with evaporative light scattering detection was developed for analysis of HPβCD. DB-67 was analyzed by HPLC with fluorescence detection.
Results
HPβCD entrapped in the aqueous compartment of liposomes was found to be membrane impermeable. Gel phase liposomes were stable in the presence of HPβCD. HPβCD complexation did not significantly alter the apparent permeability of DB67 lactone, due to its high membrane binding. However, lactone ring-opening and ionization significantly decreased the apparent permeability and improved the liposomal retention of DB-67, an effect that was amplified in the presence of 50 mM HPβCD.
Conclusions
In liposomes, cyclodextrin complexation competes with liposomal membrane binding which may temper the potential benefit of complexation in prolonging hydrophobic drug retention. Cyclodextrin complexation combined with drug ionization may nevertheless significantly enhance the retention of ionizable hydrophobic drugs in liposomes as complexation may compete more favorably with membrane binding when the drug is ionized.












Similar content being viewed by others
References
A. A. Gabizon. Liposomal drug carrier systems in cancer chemotherapy: current status and future prospects. J. Drug Target. 10:535–538 (2002). doi:10.1080/1061186021000043061.
D. D. Lasic, B. Ceh, M. C. Stuart, L. Guo, P. M. Frederik, and Y. Barenholz. Transmembrane gradient driven phase transitions within vesicles: lessons for drug delivery. Biochim Biophys Acta. 1239:145–156 (1995). doi:10.1016/0005-2736(95)00159-Z.
E. Maurer-Spurej, K. F. Wong, N. Maurer, D. B. Fenske, and P. R. Cullis. Factors influencing uptake and retention of amino-containing drugs in large unilamellar vesicles exhibiting transmembrane pH gradients. Biochim. Biophys. Acta. 1416:1–10 (1999) doi:10.1016/S0005-2736(98)00204-1.
R. P. Hertzberg, M. J. Caranfa, and S. M. Hecht. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme–DNA complex. Biochemistry. 28:4629–4638 (1989). doi:10.1021/bi00437a018.
V. Joguparthi, and B. D. Anderson. Liposomal delivery of hydrophobic weak acids: enhancement of drug retention using a high intraliposomal pH. J. Pharm. Sci. 97:433–454 (2008). doi:10.1002/jps.21135.
V. Joguparthi, T. X. Xiang, and B. D. Anderson. Liposome transport of hydrophobic drugs: gel phase lipid bilayer permeability and partitioning of the lactone form of a hydrophobic camptothecin, DB-67. J. Pharm. Sci. 97:400–420 (2008). doi:10.1002/jps.21125.
V. Joguparthi, S. Feng, and B. D. Anderson. Determination of intraliposomal pH and its effect on membrane partitioning and passive loading of a hydrophobic camptothecin, DB-67. Int. J. Pharm. 352:17–28 (2008). doi:10.1016/j.ijpharm.2007.10.003.
B. McCormack, and G. Gregoriadis. Entrapment of cyclodextrin–drug complexes into liposomes: potential advantages in drug delivery. J. Drug Target. 2:449–454 (1994). doi:10.3109/10611869408996821.
B. McCormack, and G. Gregoriadis. Comparative studies of the fate of free and liposome-entrapped hydroxypropyl-b-cyclodextrin/drug complexes after intravenous injection into rats: implications in drug delivery. Biochim. Biophys. Acta. 1291:237–244 (1996).
H. Chen, J. Gao, F. Wang, and W. Liang. Preparation, characterization and pharmacokinetics of liposomes-encapsulated cyclodextrins inclusion complexes for hydrophobic drugs. Drug Deliv. 14:201–208 (2007). doi:10.1080/10717540601036880.
D. G. Fatouros, K. Hatzidimitriou, and S. G. Antimisiaris. Liposomes encapsulating prednisolone and prednisolone–cyclodextrin complexes: comparison of membrane integrity and drug release. Eur. J. Pharm. Sci. 13:287–296 (2001) doi:10.1016/S0928-0987(01)00114-2.
Y. Hagiwara, H. Arima, Y. Miyamoto, F. Hirayama, and K. Uekama. Preparation and pharmaceutical evaluation of liposomes entrapping salicylic acid/gamma-cyclodextrin conjugate. Chem. Pharm. Bull (Tokyo). 54:26–32 (2006). doi:10.1248/cpb.54.26.
F. Maestrelli, M. L. Gonzalez-Rodriguez, A. M. Rabasco, and P. Mura. Effect of preparation technique on the properties of liposomes encapsulating ketoprofen-cyclodextrin complexes aimed for transdermal delivery. Int. J. Pharm. 312:53–60 (2006). doi:10.1016/j.ijpharm.2005.12.047.
G. Piel, M. Piette, V. Barillaro, D. Castagne, B. Evrard, and L. Delattre. Betamethasone-in-cyclodextrin-in-liposome: the effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 312:75–82 (2006). doi:10.1016/j.ijpharm.2005.12.044.
I. I. Salem, and N. Duzgunes. Efficacies of cyclodextrin-complexed and liposome-encapsulated clarithromycin against Mycobacterium avium complex infection in human macrophages. Int. J. Pharm. 250:403–414 (2003). doi:10.1016/S0378-5173(02)00552-5.
P. Hatzi, S. Mourtas, P. G. Klepetsanis, and S. G. Antimisiaris. Integrity of liposomes in presence of cyclodextrins: effect of liposome type and lipid composition. Int. J. Pharm. 333:167–176 (2007). doi:10.1016/j.ijpharm.2006.09.059.
S. L. Niu, and B. J. Litman. Determination of membrane cholesterol partition coefficient using a lipid vesicle–cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys. J. 83:3408–3415 (2002).
H. Ohvo, and J. P. Slotte. Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemistry. 35:8018–8024 (1996). doi:10.1021/bi9528816.
J. Nishijo, and H. Mizuno. Interactions of cyclodextrins with DPPC liposomes. Differential scanning calorimetry studies. Chem. Pharm. Bull. (Tokyo). 46:120–124 (1998).
G. Puglisi, M. Fresta, and C. Ventura. Interaction of natural and modified b-cyclodextrins with biological membrane model of dipalmitoylphosphatidylcholine. J. Colloid Interface Sci. 180:542–547 (1996). doi:10.1006/jcis.1996.0335.
J. Nishijo, S. Shiota, K. Mazima, Y. Inoue, H. Mizuno, and J. Yoshida. Interactions of cyclodextrins with dipalmitoyl, distearoyl, and dimyristoyl phosphatidyl choline liposomes. A study by leakage of carboxyfluorescein in inner aqueous phase of unilamellar liposomes. Chem. Pharm. Bull. (Tokyo). 48:48–52 (2000).
G. Piel, M. Piette, V. Barillaro, D. Castagne, B. Evrard, and L. Delattre. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 338:35–42 (2007). doi:10.1016/j.ijpharm.2007.01.015.
J. Fassberg, and V. J. Stella. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J. Pharm. Sci. 81:676–684 (1992). doi:10.1002/jps.2600810718.
P. S. Uster, T. M. Allen, B. E. Daniel, C. J. Mendez, M. S. Newman, and G. Z. Zhu. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 386:243–246 (1996). doi:10.1016/0014-5793(96)00452-8.
T. X. Xiang, and B. D. Anderson. Stable supersaturated aqueous solutions of silatecan 7-t-butyldimethylsilyl-10-hydroxycamptothecin via chemical conversion in the presence of a chemically modified b-cyclodextrin. Pharm. Res. 19:1215–1222 (2002). doi:10.1023/A:1019862629357.
J. R. Silvius, and M. J. Zuckermann. Interbilayer transfer of phospholipid-anchored macromolecules via monomer diffusion. Biochemistry. 32:3153–3161 (1993). doi:10.1021/bi00063a030.
Acknowledgements
This work was financially supported by a grant from NIH (NCI RO1 CA87061). VJ would like to thank Virginia Fields and Mikolaj Milewski for performing preliminary experiments in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joguparthi, V., Anderson, B.D. Effect of Cyclodextrin Complexation on the Liposome Permeability of a Model Hydrophobic Weak Acid. Pharm Res 25, 2505–2515 (2008). https://doi.org/10.1007/s11095-008-9664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9664-6