Medicinal plants play essential role in improving the human health due to the presence of bioactive constituents. Monotheca buxifolia (family Sapotaceae) leaves and fruit ethanol extracts and their fractions were subjected to biological screening for antioxidant activities. The fractions of M. buxifolia fruit extract showed better DPPH radical scavenging activity (up to 81.5% for ethyl acetate fraction) than the leaf extract and its fractions. Aqueous and hexane fractions of the leaf extract showed highest values of total antioxidant potential and total reducing power potential (388.6 ± 1.51 and 31.05 μg AAE/mg, respectively). Ethyl acetate fraction of the leaf extract contained maximum amount of phenolics (72 ± 0.41 μg GAE/mg) and flavonoids (82.59 ± 0.14 μg QE/mg). Antibacterial activity was not observed for many fractions, except a few showing minor activity against different bacterial strains. Cytotoxicity assay showed that the aqueous fraction of leaf extract had minimum cytotoxic potential with median lethal concentration (LC50) of 61.51 μg/mL. Phytotoxic behavior was checked against Trifolium protense and Brassica napus seeds. The plant possessed potent phytotoxic behavior against both plant seedlings growth. On the basis of in vitro screening, it can be suggested that M. buxifolia possesses antioxidant, antimicrobial, cytotoxic, and phytotoxic properties, which might be due to the presence of bioactive compounds. Further studies are required to isolate the major bioactive constituents and to verify both in vitro and in vivo assays.
Similar content being viewed by others
INTRODUCTION
It becomes more privilege when plant is being used in traditional medicine but still unexplored on scientific basis. Though about 80 percent of world population still depend on herbal medicines for primary healthcare needs, especially in the rural areas [1], still more than 90% of medicinal plants are not fully explored. It is also the fact that more than 50% pharmaceutical agents are either plant derived components or their derivatives. One of the important medicinal plant families is Sapotaceae, belonging to order Ericales. This family includes about 800 species of evergreen trees and shrubs in around 65 genera. Many species of this family produce edible fruits and many of them have economic value.
Monotheca buxifolia is one of important plants of this family, representing evergreen small tree having broad leaves, and is one of the most important species in hilly areas. M. buxifolia is mainly used as a fuel, fodder, and small timber, being economically valuable for local mountain inhabitant [2]. Ethnobotanically, M. buxifolia fruit is digestive, laxative, and used for treating urinary tract diseases [3]. The fruit of this species is mostly purgative, vermifuge and refrigerant. Recently it has been reported that M. buxifolia plant possesses pain, inflammation and pyrexia ameliorating properties, [4] and also exhibits selective hepatoprotective effect against antitubercular drugs [5].
Based on the importance of biological and phytochemical screening, the present study was conducted to assess M. buxifolia leaves and fruits as potential candidates for isolation of active constituents. For this purpose, the plant extracts were fractionated and screened for antioxidant, antibacterial, brine shrimp lethality cytotoxic assay, total phenolics and flavonoids, and phytotoxic assays, which will be helpful in drug development.
MATERIALS AND METHODS
Plant Collection, Extraction and Fractionation
Raw plant material (leaves and fruit) of Monotheca buxifolia were collected at Mohmand Agency Mountains, Khyber Pakhtunkhwa, Pakistan during May 2016. The plant was identified by Taxonomist Department of Plant Sciences, Quaid-i-Azam University Islamabad (voucher ID BIT-4220). The plant material was first washed and dried on filter paper. Then, the plant material was vacuum dried at 40°C for three days. Finally both parts were separately powdered in a blender.
For extraction, the powdered materials were soaked in ethanol (250 g/500 mL) and kept for three days with occasional shaking. The liquid portion was separated by Whatman filter paper and the residue was again dipped in ethanol. This process was repeated thrice and finally all respective solvent portions were combined and vacuum evaporated in a rotary evaporator at 40°C. The crude ethanol extracts were used for fractionation, and biological and phytochemical screening.
For fractionation, 100 g crude extract was suspended in 250 mL distilled water followed by liquid-liquid fractionation. First, 150 mL n-hexane was mixed with extract, shaken well, and n-hexane layer was collected on a separating funnel. This process was repeated with ethyl acetate and chloroform and finally the remaining fraction was termed as aqueous extract. Each fraction was further concentrated in a rotary evaporator and used for assays.
Antioxidant Assay
Antioxidant assay was performed with the help of 2, 2-diphenyl 1-picrylhydrazyl (DPPH) free radical scavenging, total antioxidant capacity, and reducing power assay. The antioxidant assays were performed following the established protocols as modified by Rehman, et al. [6].
The antioxidant potential was first monitored through assessing the stability of DPPH radical u under free radical scavenging. Aliquot (10 μL) of each sample prepared in DMSO (20 mg/mL) was mixed with 190 μL DPPH solution (9.2 mg/100 mL in methanol). After 30 min of incubation at room temperature, the optical absorbance was measured at 517 nm using microplate reader. The percentage radical scavenging activity (%RSA) was calculated as
where Abs is the absorbance of DPPH solution with sample and Abc is the absorbance of negative control (containing only the reagent except the sample). Ascorbic acid was used as a standard.
Phosphomolybdenum based total antioxidant capacity of the extracts and fractions was estimated by mixing 100 μL of extract/fraction (20 mg/mL in DMSO) with 1 mL of reagent (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). Ascorbic acid (1 mg/mL) was used as positive control. All mixtures were incubated on water bath at 95°C for 90 min, after which the absorbance was measured at 630 nm. The antioxidant activity was expressed in units of the amount (μg) of ascorbic acid equivalents per milligram of extract (μg AAE/mg).
The reducing power of samples was determined by previously described protocol. Concisely, 200 L (20 mg/mL DMSO) was mixed with 400 μL phosphate buffer (0.2 mol/L, pH 6.6) and 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min and trichloroacetic acid (400 μL of 10% solution) was added to the reaction mixture. The mixture was centrifuged at 3000 rpm for 10 min and 500 μL upper layer was mixed with 500 μL distilled water and 100 μL FeCl3(0.1 %) in a separate tube. The absorbance of the reaction mixture was measured at 630 nm. Blank was prepared by adding 200 μL of DMSO while ascorbic acid was used as positive control. The reducing power of each sample was expressed as μg AAE/mg extract.
Determination of Total Phenolic and Total Flavonoid Contents
The total phenolic and flavonoid contents were determined using methodology reported by Ali, et al. [7]. An aliquot of 20 μL from 20 mg/mL DMSO solution of each extract/ fraction was treated with 90 μL Folin–Ciocalteu reagent. After 5 min incubation, 90 μL sodium carbonate was added to the reaction mixture. Gallic acid was used as standard, and the absorbance was measured at 630 nm. The results were expressed as amount (μg) of gallic acid equivalents (GAE) per milligram extract (μg GAE/mg).
The total flavonoid content was determination using the aluminum chloride colorimetric method. In brief, 20 μL sample (20 mg/mL DMSO) was mixed with 10 μL each of 10% aluminum chloride and 1.0 M potassium acetate followed by addition of 160 μL distilled water. The mixture was incubated at room temperature for 30 min and the absorbance was measured at 415 nm. DMSO was used as negative control, while quercetin was positive control. The flavonoid content was expressed in amount (μg) of quercetin equivalents (QE) per milligram extract (μg QE/mg).
Antimicrobial Activity and Cytotoxicity Assay
Two types of antimicrobial assays were performed as antibacterial and antifungal assay. The antibacterial assay was accomplished against seven different bacterial strains. Two strains were Gram positive (Staphylococcus aureus ATCC 6538 and Micrococcus luteus ATCC 10240) and five strains were Gram negative (Escherichia coli ATCC 15224, Bordetella bronchiseptica ATCC 4617, Salmonella typhimurium ATCC 14028,Klebsiella pneumoniae ATCC 4619, and Enterobacter aerogens ATCC 13048). In vitro antibacterial potential of extracts was evaluated by the agar disc diffusion method as described by Bibi, et al. [1]. Alawn of fresh bacterial cultures with pre-adjusted seeding density was made on nutrient agar plates. Sterile filter paper disc impregnated with 5 μL (20 mg/mL DMSO) of each sample was placed on the seeded plates. Disc infused with cefixime served as positive control, while DMSO infused disc was used as negative control. The zone of bacterial growth inhibition was measured after incubation at 37°C for 24 h.
The brine shrimp lethality test was performed according to a previously described protocol with minor modifications proposed by Tabassum, et al. [12]. Eggs of Artemia salina were hatched in simulated sea water. The mature nauplii were harvested and transferred to each well of 96 well plate. Each extract containing ≤ 1% DMSO at final concentrations of 200, 100, 50, 25 μg/mL was then transferred to the wells containing sea water and shrimp larvae. Doxorubicin (4 mg/mL) and 1% DMSO in sea water were used as positive and negative control, respectively. After 24 h incubation period, the degree of lethality was determined by counting the number of survivors and median lethal concentration (LC50) of the test samples with ≥ 50% mortality, as calculated using tabulated curve constructed using 2D V5.01 software.
Phytotoxic Activity Assay
The phytotoxic activity was evaluated according to the method reported by Ali, et al. [8] with minor modifications. A stock solution of each test compound was prepared by dissolving 20 mg of extract in 1 mL of the corresponding solvents. From this stock solution, further dilutions (5000 ppm, 1000 ppm, and 100 ppm) were prepared. The total volume of each dilution was made up to 5 mL by adding the corresponding solvent. Autoclaved distilled water was used for blank. Phytotoxic effect of all fractions was determined on Trifolium and Brassica napus seed germination. For this purpose, one layer of Whatman no.1 filter paper was fixed on Petri plate, the plates were marked and 5 ml of the sample solution was poured onto the corresponding plate. Then, the plates were allowed to dry (evaporation of solvent). The seeds were surface sterilized with sodium hypochlorite aqueous solution (10%) for two min, washed five times with distilled water and dried with paper towel. Ten to fifteen seeds were placed in each Petri-plate and kept in the dark at room temperature (~30°C). All plates were sealed with parafilm to decrease the moisture loss. On the 3rd and 5th day, percentage of seedling germination was calculated. At the end of experiment, the fresh and dry masses were also calculated.
Statistical Analysis
All tests were performed in triplicate and the results were presented as mean ± SD. The mean values were further analyzed for least significant difference (LSD) at P ≤ 0.05 after the analysis of variance.
RESULTS AND DISCUSSION
Antioxidant Activities of Extracts
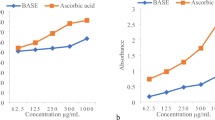
Antioxidant properties of extracts and fractions were determined through DPPH free radical scavenging activity, total antioxidant activity, and total reducing power potential. The crude extracts showed mild free radical scavenging activity (50 and 47% for the leaf and fruit extract, respectively) (Fig. 1). Fractionation process increased the activity in polar fraction. Ethyl acetate fraction of leaves and fruits showed maximum values (74.7 and 81.5% inhibition, respectively). DPPH free radical scavenging assay is considered to be a simple, quick and appropriate method of screening plant extracts for their antioxidant potential by measuring the reducing ability of samples. The DPPH test is a non-enzymatic method conventionally used to gain basic information on scavenging free radicals [9].
DPPH free radical scavenging activity, total antioxidant capacity (TAC), and total reducing power (TRP) of M. buxifolia crude extracts and their fractions. (EtOH (Lcr): Crude extract leaves, Et. Ace (Lf): Ethyl acetate fraction of leaf extract, CHCl3 (Lf): Chloroform fraction of leaf extract, n-hex (Lf): n-hexane fraction of leaf extract, Aqu (Lf): Aqueous fraction of leaf, (EtOH (Fcr): Crude extract fruit, Et. Ace Ff): Ethyl acetate fraction of fruit extract, CHCl3(Ff): Chloroform fraction of fruit extract, n-hex (Ff): n-hexane fraction of fruit extract, Aqu (Ff): Aqueous fraction of fruit). LSD test results are presented as triplicate analysis a, ab Mean difference is highly significant (P ≥ 0.000), bc slightly significant (P ≥ 0.001) and cd, d significant (P ≥ 0.05).
Phosphomolybdenum method is based on the reduction of phosphomolybdate ion in the presence of an antioxidant resulting in the formation of a green phosphate complex which is measured spectrophotometrically. Aqueous and ethyl acetate fractions of leaf extract showed maximum antioxidant capacity (301.8 ± 0.70 and 388.6 ± 1.51 g AAE/mg, respectively) (Fig. 1). Among fruit extract fractions, the aqueous fraction showed maximum antioxidant capacity (230.6 ± 0.707 μg AAE/mg). In this assay, extract reduces Mo(VI) to Mo(V) under acidic conditions that led to the creation of green colored phosphate Mo(V) complex [10].
M. buxifolia leaf and fruit extract fractions were also evaluated for the reducing power capacities that were expressed in ascorbic acid equivalent. The reducing property of sample is generally associated with the presence of reductants, which employ antioxidant action by donating a hydrogen atom and breaking the free radical chain. The n-hexane fraction of leaf extract showed highest reductive ability, followed by aqueous and ethyl acetate fractions, while in the fruit extract, ethyl acetate fraction showed highest reducing property followed by n-hexane (Fig. 1). The reducing power of M. buxifolia fractions indicates that it is likely to contribute considerably to the total antioxidant effect [11].
Oxidation in biological systems causes the release of highly reactive species such as hydroxyl and peroxy radicals. These free radicals may cause damage to DNA, proteins, phospholipids etc., which in turn may lead to vascular disease and cancer [12]. The leading contributors to the antioxidant activity are phenolic and polyphenolic compounds like flavonoids, phenolic acids and tannins. Antioxidant capability of phenols is attributed to the presence of methoxy, hydroxyl, double bond conjugation, or ketonic group in a phenolic molecule [13].
Total Phenolic and Flavonoid Contents
Isolation of natural compounds, in particular, plant-derived antioxidants has drawn the interest of researchers to treat diseases by reducing oxidative stress [14]. Total phenolic content in M. buxifolia leaves and fruit fractions expressed in gallic acid equivalents (GAE) units ranged between 72 and 10.78 μg GAE/mg. The ethyl acetate fraction of fruits exhibited maximum total phenolic content (72 ± 0.41 μg GAE/mg), whereas the phenolic content of n-hexane extract was much smaller (10.78 ± 0.75 μg AAE/mg) (Fig. 2), which is in agreement with other analogous reports [15, 16]. The antioxidant properties of compounds well correlate with total phenolic content [17] because phenols are good antioxidants and have antimutagenic and anticancer properties [18]. It has been reported that phytoconsitutents account for the hepatoprotective properties of Monotheca plant and are also involved in other activities such as anti-inflammatory, antipyretic, etc. [4, 5].
Total phenolic content (TPC) and total flavonoid content (TFC) of M. buxifolia crude extracts and fractions. (EtOH (Lcr): Crude extract leaves, Et. Ace (Lf): Ethyl acetate fraction of leaf extract, CHCl3(Lf): Chloroform fraction of leaf extract, n-hex (Lf): n-hexane fraction of leaf extract, Aqu (Lf): Aqueous fraction of leaf, (EtOH (Fcr): Crude extract fruit, Et. Ace Ff): Ethyl acetate fraction of fruit extract, CHCl3(Ff): Chloroform fraction of fruit extract, n-hex (Ff): n-hexane fraction of fruit extract, Aqu (Ff): Aqueous fraction of fruit). LSD test results are presented as triplicate analysis a, ab, b Mean difference is highly significant (P ≥ 0.000), bc, cd, c, d slightly significant (P ≥ 0.001) and de, d, e, ef, f significant (P ≥ 0.05).
The total flavonoid content was observed in a range from 82.59 to 3.15 μg QE/mg (Fig. 2). The highest amount was found in ethyl acetate fraction (82.59 ± 0.14 μg QE/mg) followed by chloroform fraction (70.63 ± 0.12 μg QE/mg). Literature reports that plants contain high amount of flavonoids [19]. The results suggest that phenolics and flavonoids may be the main contributors to antioxidant properties and inhibitory action toward the oxidative reactions. Flavonoids are also known as neuroprotective and anti-inflammatory molecules [20]. High amounts of phenolic compounds are indicative of high antioxidant and reducing power activities [21]. Positive correlation has been found between the reducing power, antioxidant capacity, total phenolic and total flavonoid contents, specifically in moderately polar solvents, which is in good agreement with reported data [22].
Antibacterial and Cytotoxic Assay
Antibacterial assay was carried out against seven bacterial strains. Crude extract and their fractions showed less significant antibacterial activity. Ethyl acetate and aqueous fractions of leaf extract exhibited 11 mm zone of inhibition against M. Leuteus and B. bronchiseptica, respectively (Table 1). Cytotoxic analysis using brine shrimp is cost effective, quick and feasible to carry out cytotoxic examination [23, 24]. Significant cytotoxic activity was observed by Aqueous fraction of leaf (LC50= 61.51 μg/ml) (Fig. 3); followed by n-hexane (LC50= 125 μg/ml). Leaf extract and fractions demonstrated higher cytotoxic activity. Significant cytotoxic behavior is helpful to provide cellular protection against cell necrosis and act as immunological parameter to monitor disease progression [25]. The degree of lethality is normally presumed directly proportional to the potency of extract. Brine shrimp cytotoxicity assay is widely used as initial procedure for exploration of antimicrobial, antitumor, antifungal, antimalarial, molluscicidal, larvicidal and insecticidal activities [26].
Cytotoxic activity of M. buxifolia crude extracts and fractions. (EtOH (Lcr): Crude extract leaves, Et. Ace (Lf): Ethyl acetate fraction of leaf extract, CHCl3(Lf): Chloroform fraction of leaf extract, n-hex (Lf): n-hexane fraction of leaf extract, Aqu (Lf): Aqueous fraction of leaf, (EtOH (Fcr): Crude extract fruit, Et. Ace Ff): Ethyl acetate fraction of fruit extract, CHCl3(Ff): Chloroform fraction of fruit extract, n-hex (Ff): n-hexane fraction of fruit extract, Aqu (Ff): Aqueous fraction of fruit). LSD test results are presented as triplicate analysis a, ab, b Mean difference is highly significant (P ≥ 0.000), bc, cd, c, d slightly significant (P ≥ 0.001) and de, d, e, ef, f significant (P ≥ 0.05).
Phytotoxic Activity
Phytotoxic (allelopathic) effect of M. buxifolia was evaluated against Trifolium protense and Brassica napus growth under controlled environmental condition in growth room. Results demonstrated that all fractions of Monotheca plant have great effect on growth of seedling of Trifolium plant. In particular, seedling growth was markedly inhibited by ethyl acetate fraction at all three concentrations studied. Total fresh weight and dry weight of all groups were observed and found that ethyl acetate was more effective as compared to other fractions on the 3rd and 5th days of treatment at 100 ppm, 1000 ppm and 5000 ppm. The activity against B. napus was stronger as compared to Trifolium plant. The ethyl acetate fraction showed maximum (about 90%) inhibitory effect on the seedling growth of B. napus. Total fresh weight and dry weight of all groups were observed and found that ethyl acetate and chloroform was most effective as compared to control and other fractions.
The seed germination inhibition potential might be associated with the presence of allelochemicals that affect germination and growth of plants [27, 28]. It has been reported that maximum allelopathic suppression may occur when allelochemicals interfere with early growth stages of plant. The extracts affected germination and weight associated parameters in concentration dependent manner [29]. The observed variations in fresh weight and dry weight indicate that the plant extracts varied water holding capacity of plantlets. The identification of compounds responsible for causing allelopathy is of great interest as it can be used as an effective alternative method for weed control. Allelochemicals mainly function by affecting cell division or by suppressing enzymes responsible for the movement of nutrients, important for germination [30]. The current focus of agriculture industry is to find a safe and biological solution to reduce foreseen hazardous impacts of herbicides and insecticides [31].
There was no previous reports about phytotoxic behavior of M. buxifolia. Many studies reported growth inhibition or germination of certain plants like alfalfa by alkaloidal content of other plants. In this regard, M. buxifolia is of great importance since it showed more pronounced allelopathic effects. The phytotoxic activity of the plant is due to the existence of phytotoxic substances in different parts of plant that leak out under natural conditions [32].
Further investigations involving bioassay guided isolation of these crude extracts is planned for complete exploitation of the plant.
References
Y. Bibi, S. Nisa, F. M. Chaudhary, and M. Zia, BMC Comp. Med., 11, 52 (2011).
A. Yahyai and A. Nabhani, 27 International Horticulture Exhibitions, ABS, Korea (2006).
A. Rashid and S. Khan, J. Chem. Sci.,52, 1026 – 1031 (2014).
I. Ullah, J. A. Khan, M. Shahid, et al., BMC Comp. Alt. Med., 16(1), 273 (2016).
I. Ullah, J. A. Khan, A. Adhikari, and M. Shahid, Bangladesh J. Pharmacol., 11(1), 248 – 56 (2016).
R. Rehman, M. Chaudhary, K. Khawar, et al., Biologia, 69, 341 – 349 (2014).
J. S. Ali, I. ul Haq, A. Ali, et al., Cogent Bio., 3, 1283875 (2017).
A. Ali, A. R. Phull, M. Zia, et al., Environ. Nanotechnol. Monitor. Manag., 4, 74 – 84 (2015).
R. Prior, X. Wu, and K. Schaich, J. Agric. Food Chem., 53, 4290 – 4303 (2005).
P. Prieto and M. Pineda, Aguilar M. Biochem., 269, 23 – 371 (1999).
S. Jan, M. R. Khan, U. Rashid, and J. Bokhari, Osong Public Health Res. Pers. 4, 246 – 254 (2013).
S. Tabassum, B. Mirza, G. M. Khan, et al., BMC Com. Alt. Med., 17, 146 (2017).
L. Tapsell. Med. J. Australia, 185, 4 – 2 (2006).
A. Luximon-Ramma, et al. J. Agric. Food Chem., 50, 5042 – 5047 (2002).
S. Sahreen, M. Khan, R. Khan, J. Food Chem., 122, 12 – 45 (2010).
C. Ao, J. Food Contr., 10, 9408 (2008).
Y. Velioglu, G. Mazza, and L. Gao, J. Agric. Food Chem., 46, 41 – 13 (1998).
N. Ahmad and H. Mukhtar, Nutr. Rev., 57, 78 – 83 (1999).
B. Halliwell, Arch. Biochem. Biophys., 476, 10 – 12 (2008).
N. Cho, et al., Food Chem. Toxicol., 58, 355 – 361 (2013).
M. Viuda-Martos, et al., Flavor Fragr J., 25, 13 – 19 (2010).
M. M. Özcan, Ö. Erel, and E. E. Herken, J. Med. Food, 12, 198 – 202 (2009).
B. Kivack, T. Mert, and H. Tansel, Turkish J. Biol., 26, 197 – 200 (2001).
L. Carballo, L. Hernandez, P. Perzer, and M. J. Gravalos, Bio. Med. Central., 2, 1 – 10 (2002).
Y. Mizutani, Y. Okada, and B. Bonavida, Cancer Biother. Radiopharm., 11, 385 – 91 (1996).
E. C. Silva, et al., Mem. Inst. Oswaldo Cruz, 104, 48 – 56 (2009).
M. Evenari, Bot. Rev., 15, 153 – 194 (1949).
J. B. Gressel and L. G. Holm, Weed Res.,4, 44 – 53 (1964).
Inderjit, K. I. Keating, Adv. Agron., 67, 141 – 231 (1999).
U. Batlang and D. D. Shushu, J. Agron.,6, 541 – 547 (2007).
T. D. Khanh, M. I. Chung, T. D. Xuan, and S. Tawata, J. Agron. Crop Sci., 191, 172 – 184 (2005).
A. Tawaha and M. Turk, J. Agron. Crop Sci., 189, 298 – 303 (2003).
ACKNOWLEDGMENTS
Authors are thankful to Higher Education Commission Pakistan for funding (NRPU-20-4220) of this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, I., Ali, J.S., Ul-Haq, I. et al. Biological and Phytochemicals Properties of Monotheca buxifolia: An Unexplored Medicinal Plant. Pharm Chem J 54, 293–301 (2020). https://doi.org/10.1007/s11094-020-02194-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02194-y