Abstract
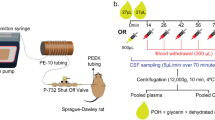
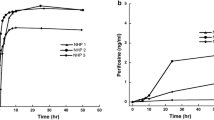
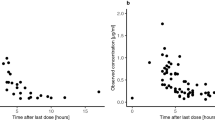
The blood–brain barrier (BBB) limits entry of most chemotherapeutic agents into the CNS, resulting in inadequate exposure within CNS tumor tissue. Intranasal administration is a proposed means of delivery that can bypass the BBB, potentially resulting in more effective chemotherapeutic exposure at the tumor site. The objective of this study was to evaluate the feasibility and pharmacokinetics (plasma and CSF) of intranasal delivery using select chemotherapeutic agents in a non-human primate (NHP) model. Three chemotherapeutic agents with known differences in CNS penetration were selected for intranasal administration in a NHP model to determine proof of principle of CNS delivery, assess tolerability and feasibility, and to evaluate whether certain drug characteristics were associated with increased CNS exposure. Intravenous (IV) temozolomide (TMZ), oral (PO) valproic acid, and PO perifosine were administered to adult male rhesus macaques. The animals received a single dose of each agent systemically and intranasally in separate experiments, with each animal acting as his own control. The dose of the agents administered systemically was the human equivalent of a clinically appropriate dose, while the intranasal dose was the maximum achievable dose based on the volume limitation of 1 mL. Multiple serial paired plasma and CSF samples were collected and quantified using a validated uHPLC/tandem mass spectrometry assay after each drug administration. Pharmacokinetic parameters were estimated using non-compartmental analysis. CSF penetration was calculated from the ratio of areas under the concentration–time curves for CSF and plasma (AUCCSF:plasma). Intranasal administration was feasible and tolerable for all agents with no significant toxicities observed. For TMZ, the degrees of CSF drug penetration after intranasal and IV administration were 36 (32–57) and 22 (20–41)%, respectively. Although maximum TMZ drug concentration in the CSF (Cmax) was lower after intranasal delivery compared to IV administration due to the lower dose administered, clinically significant exposure was achieved in the CSF after intranasal administration with the lower doses. This was associated with lower systemic exposure, suggesting increased efficiency and potentially lower toxicities of TMZ after intranasal delivery. For valproic acid and perifosine, CSF penetration after intranasal delivery was similar to systemic administration. Although this study demonstrates feasibility and safety of intranasal drug administration, further agent-specific studies are necessary to optimize agent selection and dosing to achieve clinically-relevant CSF exposures.





Similar content being viewed by others
References
Abbott NJ (2013) Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3):437–449
Pasha S, Gupta K (2010) Various drug delivery approaches to the central nervous system. Expert Opin Drug Deliv 7(1):113–135
Vanan MI, Eisenstat DD (2015) DiPG in children–what can we learn from the past? Front Oncol 5:237
Warren KE (2013) Novel therapeutic delivery approaches in development for pediatric gliomas. CNS Oncol 2(5):427–435
Miyake MM, Bleier BS(2015) The blood–brain barrier and nasal drug delivery to the central nervous system. Am J Rhinol Allergy 29(2):124–127
Pires A et al (2009) Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci 12(3):288–311
Costantino HR et al (2007) Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm 337(1–2):1–24
Panagiotou I, Mystakidou K (2010) Intranasal fentanyl: from pharmacokinetics and bioavailability to current treatment applications. Expert Rev Anticancer Ther 10(7):1009–1021
Bitter C, Suter-Zimmermann K, Surber C (2011) Nasal drug delivery in humans. Curr Probl Dermatol 40:20–35
Mittal D et al (2014) Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv 21(2):75–86
Djupesland PG (2013) Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res 3(1):42–62
Attkins NJ et al (2009) Predictability of intranasal pharmacokinetics in man using pre-clinical pharmacokinetic data with a dopamine 3 receptor agonist, PF-219061. Xenobiotica 39(7):523–533
Dhuria SV, Hanson LR, Frey WH 2nd (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99(4):1654–1673
Lochhead JJ, Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64(7):614–628
Illum L (2000) Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11(1):1–18
Johnson NJ, Hanson LR, Frey WH (2010) Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm 7(3):884–893
Merkus FW, van den Berg MP (2007) Can nasal drug delivery bypass the blood–brain barrier?: questioning the direct transport theory. Drugs R D 8(3):133–144
Thorne RG et al (2008) Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152(3):785–797
van Woensel M et al (2013) Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers (Basel) 5(3):1020–1048
Shingaki T et al (2010) Transnasal delivery of methotrexate to brain tumors in rats: a new strategy for brain tumor chemotherapy. Mol Pharm 7(5):1561–1568
Djupesland PG, Messina JC, Mahmoud RA (2014) The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv 5(6):709–733
Hashizume R et al (2008) New therapeutic approach for brain tumors: intranasal delivery of telomerase inhibitor GRN163. Neuro Oncol 10(2):112–120
Peterson A et al (2014) A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option. J Neurooncol 116(3):437–446
CO, D.A.F. et al (2013) Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res 33(12):5625–5631
Patel M et al (2003) Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol 61(3):203–207
Stapleton SL et al (2008) Plasma and cerebrospinal fluid pharmacokinetics of valproic acid after oral administration in non-human primates. Cancer Chemother Pharmacol 61(4):647–652
Cole DE et al (2015) Plasma and cerebrospinal fluid pharmacokinetics of the Akt inhibitor, perifosine, in a non-human primate model. Cancer Chemother Pharmacol 75(5):923–928
Albus U (2012) Guide for the care and use of laboratory animals (8th edn). Lab Anim 46(3):267–268
Mygind N, Upper airway: structure, function and therapy, in in aerosol in medicine. Princples, diagnoses, and therapy. 1985, Elsevier, Amsterdam, pp 1–20
Peer CJ et al (2016) Quantification of temozolomide in nonhuman primate fluids by isocratic ultra-high performance liquid chromatography-tandem mass spectrometry to study brain tissue penetration following intranasal or intravenous delivery. Separations 3(1):4
Harkema JR (1990) Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ Health Perspect 85:231
Acknowledgements
This work was presented in part at the 2015 Society of Neuro-Oncology and the Society of CNS Interstitial Delivery of Therapeutics (SNO-SCIDOT) Joint Conference on Therapeutic Delivery to the CNS in San Antonio, TX. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Disclaimer
The views expressed in this article are those of the author(s) and do not reflect the official policy or views of the National Cancer Institute, the National Institutes of Health, the U.S. Department of Health and Human Services, the Department of the Army/Navy/Air Force, Department of Defense, or any other agency of the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors James C. League-Pascual, Cynthia M. Lester-McCully, Shaefali Shandilya, Lukas Ronner, Louis Rodgers, Rafael Cruz, Cody J. Peer, William D. Figg, and Katherine E. Warren all individually declare no conflict of interest.
Research involving human and animal rights
The National Cancer Institute Animal Care and Use Committee approved this study. All applicable institutional guidelines for the care and use of animals were followed. This study does not contain any studies with human participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
League-Pascual, J.C., Lester-McCully, C.M., Shandilya, S. et al. Plasma and cerebrospinal fluid pharmacokinetics of select chemotherapeutic agents following intranasal delivery in a non-human primate model. J Neurooncol 132, 401–407 (2017). https://doi.org/10.1007/s11060-017-2388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2388-x