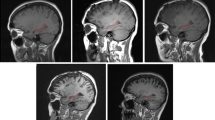
Abstract
Bevacizumab is widely used for treatment of high-grade gliomas and other malignancies. Because bevacizumab has been shown to be associated with neurocognitive decline, this study is designed to investigate whether prolonged treatment with bevacizumab is also associated with brain atrophy. We identified 12 high-grade glioma patients who received bevacizumab for 12 months at the first recurrence and 13 matched controls and blindly compared the volumes of the contralateral hemispheres and contralateral ventricle in these two groups at baseline and after 12 ± 2 months of the baseline scan by two independent analyses. The volumes of the contralateral hemispheres and ventricles did not differ significantly between the two groups at baseline. Whereas, in the control group the volumes of the contralateral hemisphere changed subtly from baseline to follow-up (p = 0.23), in the bevacizumab-treated group the volumes significantly decreased from baseline to follow-up (p = 0.03). There was significant increase in the contralateral ventricle volume from base line to follow-up scans in both the control group (p = 0.01) and in the bevacizumab group (p = 0.005). Both the absolute and the percentage changes of contralateral hemisphere volumes and contralateral ventricular volumes between the two patient groups were statistically significant (p < 0.05). Results of this study demonstrate prolonged treatment with bevacizumab is associated with atrophy of the contralateral brain hemisphere.


Similar content being viewed by others
References
Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA (2006) Vascular endothelial growth factor localization in the adult. Am J Pathol 168:639–648
Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E (2010) VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development 137:261–271. doi:10.1242/dev.039636
Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E (2011) Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A 108:5081–5086. doi:10.1073/pnas.1007640108
Bengoetxea H, Argandona EG, Lafuente JV (2008) Effects of visual experience on vascular endothelial growth factor expression during the postnatal development of the rat visual cortex. Cereb Cortex 18:1630–1639
Acker T, Beck H, Plate KH (2001) Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev 108:45–57
Barouk S, Hintz T, Li P, Duffy AM, MacLusky NJ, Scharfman HE (2011) 17beta-estradiol increases astrocytic vascular endothelial growth factor (VEGF) in adult female rat hippocampus. Endocrinology 152:1745–1751. doi:10.1210/en.2010-1290
Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS (2009) The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Investig 119:3373–3383. doi:10.1172/JCI39378
Licht T, Keshet E (2013) Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci 70:1727–1737. doi:10.1007/s00018-013-1280-x
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36:827–835
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A 99:11946–11950
Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG (2004) Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol 14:237–248
Gao P, Shen F, Gabriel RA, Law D, Yang E, Yang GY, Young WL, Su H (2009) Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke 40:3596–3600. doi:10.1161/STROKEAHA.109.561050
Louissaint A Jr, Rao S, Leventhal C, Goldman SA (2002) Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960
Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA (2007) VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res 85:740–747
Adamcio B, Sperling S, Hagemeyer N, Walkinshaw G, Ehrenreich H (2010) Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Behav Brain Res 208:80–84. doi:10.1016/j.bbr.2009.11.010
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF (2001) Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol 158:1145–1160
van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N (1999) VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Investig 104:1613–1620
Salmaggi A, Eoli M, Frigerio S, Silvani A, Gelati M, Corsini E, Broggi G, Boiardi A (2003) Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol 62:297–303
Schmidt NO, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen EM, Lamszus K (1999) Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer 84:10–18
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, Park M, Bergers G (2012) VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22:21–35. doi:10.1016/j.ccr.2012.05.037
Soda Y, Myskiw C, Rommel A, Verma IM (2013) Mechanisms of neovascularization and resistance to anti-angiogenic therapies in glioblastoma multiforme. J Mol Med 91:439–448. doi:10.1007/s00109-013-1019-z
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Eng J Med 370:699–708. doi:10.1056/NEJMoa1308573
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 352:987–996. doi:10.1056/NEJMoa043330
Kleihues PBP, Aldape Kd et al (2007) WHO classification of tumors of the central nervous system. IARC Press, Lyon
Bag AK, Cezayirli PC, Davenport JJ, Gaddikeri S, Fathallah-Shaykh HM, Cantor A, Han XS, Nabors LB (2014) Survival analysis in patients with newly diagnosed primary glioblastoma multiforme using pre- and post-treatment peritumoral perfusion imaging parameters. J Neurooncol 120:361–370. doi:10.1007/s11060-014-1560-9
Linkous AG, Yazlovitskaya EM (2011) Angiogenesis in glioblastoma multiforme: navigating the maze. Anticancer Agents Med Chem 11:712–718
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z, Bartek J (2008) Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle 7:2553–2561
Hoelzinger DB, Demuth T, Berens ME (2007) Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 99:1583–1593
Welti J, Loges S, Dimmeler S, Carmeliet P (2013) Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Ivestig 123:3190–3200. doi:10.1172/JCI70212
Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13:871–882. doi:10.1038/nrc3627
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Eng J Med 370:709–722. doi:10.1056/NEJMoa1308345
Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S (2005) Dehydration confounds the assessment of brain atrophy. Neurology 64:548–550. doi:10.1212/01.WNL.0000150542.16969.CC
Rao AB, Richert N, Howard T, Lewis BK, Bash CN, McFarland HF, Frank JA (2002) Methylprednisolone effect on brain volume and enhancing lesions in MS before and during IFNbeta-1b. Neurology 59:688–694
Li SF, Sun YB, Meng QH, Li SR, Yao WC, Hu GJ, Li ZJ, Wang RZ (2009) Recombinant adeno-associated virus serotype 1-vascular endothelial growth factor promotes neurogenesis and neuromigration in the subventricular zone and rescues neuronal function in ischemic rats. Neurosurgery 65:771–779. doi:10.1227/01.NEU.0000349931.61771.52
Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, Kim EJ, Choi JS, Kim S, Rhim H, Kaang BK, Son H (2008) Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal 20:714–725. doi:10.1016/j.cellsig.2007.12.009
Ruiz de Almodovar C, Coulon C, Salin PA, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, Van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan BF, Eriksson U, Moons L, D’Hooge R, Haigh JJ, Belin MF, Schiffmann S, Van Hecke P, Gallez B, Vinckier S, Chedotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C (2010) Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci 30:15052–15066. doi:10.1523/JNEUROSCI.0477-10.2010
Conflict of interests
AKB: Consultant to Dotarem Advisory board. Guerbet LLc. HK: No disclosure. YG: No disclosure. MB: No disclosure. PPW: No disclosure. HMF: No disclosure. DG: No disclosure. JMM: Leadership role, Stocks, Consultancy, Intellectual property and Research Funding: Catherex Inc. & Aettis Inc.; Research funding: ICT. JF: Research funding: Varian Oncology. TMB: No disclosure. AK: No disclosure. GKF: No disclosure. PRC: No disclosure. LBN: Consultant, Merck Inc.; Travel allowance: BMS. XH: No disclosure.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1751_MOESM3_ESM.tiff
Box plots showing contralateral hemisphere and ventricle volumes from the initial analysis. (A, B) Contralateral hemisphere volumes at baseline and follow-up scans of patients treated with (A) combination of bevacizumab and standard of the care (labeled as Bevacizumab) or (B) only standard of the care (labeled as control). (C, D) Contralateral ventricle volumes at baseline and follow-up scans of patients treated with (A) combination of bevacizumab and standard of the care or (B) only standard of the care. Supplementary material 3 (TIFF 332 kb)
11060_2015_1751_MOESM2_ESM.tiff
3D volume renderings of contralateral hemisphere and ventricle at baseline and follow-up scans of two representative patients treated with (A) combination of bevacizumab and standard of the care (labeled as Bevacizumab) and (B) only standard of the care (labeled as Control). Same size scale was applied for all hemispheres (or ventricles). This image is from the initial analysis. Supplementary material 2 (TIFF 428 kb)
Rights and permissions
About this article
Cite this article
Bag, A.K., Kim, H., Gao, Y. et al. Prolonged treatment with bevacizumab is associated with brain atrophy: a pilot study in patients with high-grade gliomas. J Neurooncol 122, 585–593 (2015). https://doi.org/10.1007/s11060-015-1751-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1751-z