Abstract
Regulatory T-cells play an important role in the regulation of the immune response and the mediation of dominant immunologic tolerance. We have previously shown that these cells are elevated in tumors and blood of patients with glioblastoma multiforme. Heme oxygenase-1, a rate-limiting enzyme in heme catabolism, has also been shown to accumulate during glioma progression and to play a critical role in FoxP3 mediated immune suppression. In this study, we investigated the correlation between FoxP3 and HO-1 expression in patients with various grades of astrocytoma (WHO grade II–IV). Using qualitative and quantitative reverse transcriptase–polymerase chain reaction and quantitative flow cytometry analyses, we analyzed FoxP3 and HO-1 expression in 19 patients with different grades of astrocytoma. We observed the highest level of FoxP3 expression in patients with grade IV tumors (11.54 ± 1.95%) vs. grade III (6.74 ± 0.19%) or grade II (2.53 ± 0.11%) (P < 0.05). Moreover, in grade IV tumors, the frequency of HO-1 mRNA expression in CD4+CD25+ cells was 11.8 ± 2.45% vs. 7.42 ± 0.31% in grade III and 2.33 ± 0.12% in grade II. Tumor infiltrating Treg stained positively with anti-HO-1 antibody. The expression of HO-1 correlated with CD4+CD25+FoxP3+ infiltration (r = 0.966). Our results confirm that HO-1 expressing Treg accumulate during glioma progression. This study also suggests that HO-1 mRNA expression is linked to the induction of Foxp3 in CD4+CD25+ glioma infiltrating Treg. These findings support the suppressive role played by regulatory T-cells in the growth of malignant brain tumors.
Similar content being viewed by others
Introduction
Regulatory T-cells (also known as CD4+CD25+FoxP3+, Treg) are a specialized subpopulation of T-cells that act to suppress activation of the immune system and thereby maintain immune system homeostasis and tolerance to self. There is increasing evidence for the existence of elevated numbers of regulatory T-cells in solid tumors and hematological malignancies [1–10]. The presence of infiltrating Treg may be detrimental to the host defense against the tumor, while the presence of effector T lymphocytes, including CD8+ and non-regulatory CD4+ helper T-cells may be beneficial. We have previously shown that there is an increase in the number of Treg both in tumor infiltrating lymphocytes as well as peripheral blood of patients with glioblastoma multiforme (GBM) [11]. Moreover, we have also shown that depletion of Treg prolongs the survival of mice with experimental brain tumors [12].
Heme oxygenase (HO) is the rate-limiting enzyme in the catabolism of heme to biliverdin, carbon monoxide (CO), and free iron. To date, three isoforms of mammalian HO have been identified: HO-1, HO-2, and HO-3 [13]. HO-1 is inducible by a variety of stimuli, in particular, oxidative stress [14, 15]. HO-2 and HO-3 are constitutive iosozymes, found ubiquitously throughout the body, with high expression in brain and testes [16, 17]. Induction of HO-1 expression has been associated with neuroprotection during hyperthermia in glial cells [18] and during hypoxia [19]. HO-1 expression has also been observed in a wide range of experimental diseases of the rodent brain such as traumatic brain injury [20], ischemia [21], and human Alzheimer’s disease [22]. In brain tumors, elevated HO-1 expression has been observed [23, 24], and in at least one report, it would appear that HO-1 accumulates during oligodenodroglioma progression [25].
The exact functional role of HO-1 expression is not fully understood in T-cells. However, growing evidence has shown that human Treg constitutively express HO-1 and that HO-1 inhibits T-cell proliferation [26]. Over-expression of HO-1 further renders T-cells resistant to Fas-mediated apoptosis [27]. Most recently, FoxP3, which encodes a forkhead/winged-helix transcription repressor specifically expressed in Treg, has been shown to induce HO-1 expression [28]. Taken together, these studies suggest that HO-1 may be an important effector of FoxP3-mediated immune suppression and an important target for further clinical development.
In the present study, we investigated the expression of HO-1 and Treg infiltration in various grades of human glioma. Both qualitative and quantitative reverse transcription polymerase chain reaction was used to demonstrate HO-1 and FoxP3 gene expression. Flow cytometry was used to quantify Treg infiltration. We report here for the first time that HO-1 and FoxP3 expression directly correlates with tumor grade in patients with malignant glioma.
Materials and methods
Patient samples
Resected specimens from 19 patients who underwent operations in the Division of Neurosurgery at the University of Chicago from 2004 to 2006 were evaluated in this study. According to the WHO classification, tumors included three grade II astrocytomas, four grade III astrocytomas, and 12 grade IV astrocytomas (Table 1). Healthy brain controls (n = 4) consisted of surgical specimens obtained during temporal lobe resections for seizure focus. All patients received perioperative steroids. Histological confirmation of the diagnosis, for both tumors and normal cortex, was obtained in all cases. Of note, only normal and healthy brain rather than diseased tissue, was used for controls. All surgical specimens were analyzed immediately after removal. This study was approved by the Institutional Review Board of the University of Chicago.
Cell sorting
The separation of CD4+CD25− cells from CD4+CD25+ cells from brain tumor samples was done by positive selection using Treg Isolation Kit (Miltenyi Biotec, Auburn, CA). Briefly, 1 × 107 cells were resuspended in 80 μl buffer and the cells were magnetically labeled by adding 20 μl of anti-CD25 microbeads. The cells were incubated for 15 min and then washed by centrifugation and resuspeded in 1 ml of buffer per 1.25 × 108 cells. CD4+CD25+ regulatory T-cells were isolated by positive selection with MS Columns. The effluent was collected; the purity of cells was analyzed by flow cytometry and was more than 90%.
Multi-color flow cytometry analysis
One million tumor infiltrating lymphocytes (TIL) were resuspended in staining buffer (3% FBS in PBS). Tumor cell suspensions were prepared by homogenization of the tumor with a cell strainer and washing of the cells with staining buffer. Erythrocytes were lysed and cells were then stained with various antibodies. Monoclonal antibodies to CD3, CD4, CD25, and Foxp3 were obtained from BD Biosciences (San Jose, CA). The analysis of CD4+CD25+ T-cells was performed as a percentage of total CD3+ cells in TILs. Monoclonal antibody to HO-1 was from Stressgen Biotechnologies (Victoria, BC). These monoclonal antibodies were directly coupled to fluorescein isothiocyanate, phycoerythrin, or phycoerythrin-indotricarbocyanin. Antibodies were used at 5 μg/ml and staining was done in fluorescence-activated cell sorting (FACS) buffer on ice for 45 min. Surface marker expression on TIL cells was visualized with FACSCaliburTM (BD Biosciences) and was analyzed with FlowJo software (BD Biosciences). Tumor cells sorting without tumor infiltrating lymphocyte was done with a MoFlo cell sorter (Cytomation, Fort Collins, CO).
Semiquantitative RT-PCR and real-time PCR
Total cellular RNA was isolated using RNeasy kit (Ambion Inc. Austin, TX) according to the manufacturer’s protocol. Equivalent amounts of RNA were reverse-transcribed with the superscript II-RT kit (Invitrogen). The cDNA amplification was analyzed quantitatively by incorporation of SYBR Green (Applied Biosystems) into double stranded DNA. PCR was done in a total volume of 25 μl containing 1 μl cDNA template, 0.5 μM of each primer, and SYBR Green Master Mix (Applied Biosystems, Foster City, CA) under the following conditions: 15 min hot start at 95°C, 15 s denaturation at 95°C, 20 s annealing of primers at 54°C, and 15 s elongation at 72°C, for 32–35 cycles. Gene expression was analyzed with the ABI PRISM 7300 Sequence Detection Software version 1.9.1 (Applied Biosystems). Sample results were normalized by dividing the value of the unknown gene by the value of the endogenous reference gene encoding glyceraldehyde phosphate dehydrogenase (GAPDH). Quadruplicate reactions were done with all cDNA samples. We used the following primers (Invitrogen, Carlsbad, CA): HO-1 forward (5′-ACT TTC AGA AGG GTC AGG TGT CC-3′); HO-1 reverse (5′-TTG AGC AGG AA G GCG GTC TTA G-3′); Foxp3 forward (5′-ATG CCT CCT CTT CTT CCT TGA-3′); Foxp3 reverse (5′-ATT GTG CCC TGC CCT TCT CA-3′); GAPDH forward (5′-GGC ATG GAC TGT GGT CAT GA-3′), and GAPDH reverse (5′-TGC ACC ACC AAC TGC TTA GC-3′). HO-1 and Foxp3 mRNA levels were normalized to total CD3+ T-cells.
Statistical analysis
Statistical analysis was performed with StatView software (SAS). Differences in continuous variables between groups were tested by ANOVA with the Scheff ‘ F-test used as a post hoc test. Correlation was analyzed by multiple linear regression method. The observed differences were considered statistically significant if P was less than 0.05. All data are expressed as mean ± SEM.
Results
CD4+CD25+ T-cell infiltration increases with tumor grade
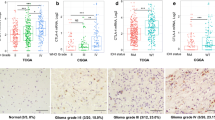
The CD4+CD25+ T-cells were gated via the forward and side scatter properties of TIL (Fig. 1A). Analysis of TIL from glioma patients shows an increase in CD4+CD25+ frequency with increasing tumor grade (Fig. 1B). The percentage of Treg was 11.33 ± 1.52% (range, 10.46–13.5%) in grade II, 15.75 ± 1.5% (range, 14.88–17.5%) in grade III, and 24.85% ± 2.67% (range, 20–28.4%) in grade IV. The observed increase in Treg between grades II and III glioma as well as grades III and IV was statistically significant (P < 0.02).
Frequency of tumor infiltrating lymphocytes (TIL) in patient with astrocytoma (grade II–IV). (A) Treg infiltration in human astrocytoma was examined by flow cytometry analysis of CD4 and CD25 expression of electronically gated TIL cells. (B) Figure B shows the frequency of Treg with grade of disease. The mean frequency of regulatory T-cells was 11.33 ± 1.52% (range: 10.46–13.5%) in grade II, 15.75 ± 1.5% (range: 14.88–17.5%) in grade III, and 24.85 ± 2.67% (range: 20–28.4%) in grade IV glioma tumors (P < 0.02) in total CD3+ T-cells
Tumor infiltrating CD4+CD25+ T-cells express FoxP3
Given that FoxP3 is a marker for Treg cells, we examined FoxP3 mRNA expression in sorted CD4+CD25+ and CD4+CD25− in different tumor grades. First, FoxP3 mRNA expression was analyzed by semiquantitative RT-PCR and then quantified by real-time PCR. As shown in Fig. 2, the expression of FoxP3 mRNA increased with tumor grade. In fact, we observed the highest level of FoxP3 expression in patients with grade IV tumors (11.54 ± 1.95; range [8.12–13.74%]) vs. grade III (6.74 ± 0.19%; range [6.61–6.88%]) or grade II (2.53 ± 0.11%; range [2.54–2.61%]) (P < 0.05), confirming that CD4+CD25+FoxP3+ T-cells preferentially accumulate in high-grade tumors.
Foxp3 expression in Treg from glioma patients compared to glioma tumor sorted without TIL or healthy brain controls. (A) RT-PCR of FoxP3 mRNA purified from isolated CD4+CD25+, CD4+CD25− T-cells, sorted tumor cells without TIL, or healthy brain. The reaction was positive in CD4+CD25+ isolated from patients at different stage of disease, but negative in CD4+CD25− T-cells, sorted tumor cells without TIL, and healthy brain. (B) Foxp3 quantification in purified CD4+CD25+ in different tumor grades. The level of FoxP3 expressed is grade IV tumors was higher (11.54 ± 1.95%; range: 8.12–13.74%) compared to grade III (6.74 ± 0.19%; range: 6.61–6.88%) or grade II (2.53 ± 0.11%; range: 2.54–2.61%) (P < 0.05). Of note, there was a total absence of FoxP3 expression in healthy brain and sorted tumor cells. GAPDH expression was used to normalize gene expression. Relative expression was calculated for FoxP3 gene by using ΔCT method. The data shown is the mean ± SD
Expression of HO-1 depends on the presence of tumor infiltrating CD4+CD25+FoxP3+ T-cells
To determine whether the reported expression of HO-1 in brain tumors is a function of tumors cells or the presence of regulatory T-cells, we examined HO-1 expression in both tumor cells and glioma infiltrating lymphocytes. The expression of HO-1 was first analyzed by semi-quantitative RT-PCR. As shown in Fig. 3A, HO-1 mRNA was detected in all glioma samples. However, the detection of HO-1 depended on the presence of tumor infiltrating lymphocytes as no HO-1 was detected in sorted tumor cells alone. Furthermore, real-time PCR demonstrated a significant increase in HO-1 mRNA expression (P < 0.05) depending on the tumor grade. In grade IV tumors, the frequency of HO-1 mRNA expression in CD4+CD25+ cells was 11.8 ± 2.45% (range: 8.14–15.33%) vs. 7.42 ± 0.31% (range: 7.2–7.65%) in grade III, and 2.33 ± 0.12% (range: 2.25–2.42%) in grade II (Fig. 3B).
HO-1 expression in Treg from glioma patients compared to glioma tumor sorted without TIL or healthy brain controls. (A) RT-PCR of HO-1 mRNA prepared from purified CD4+CD25+, CD4+CD25− T-cells, sorted tumor cells without TIL, and healthy brain. The reaction was positive in CD4+CD25+ isolated from patients at different stage of disease, but negative in CD4+CD25− T-cells, sorted tumor cells without TIL, or healthy brain. (B) HO-1 quantification in isolated CD4+CD25+ in different tumor grades. The level of HO-1 expressed in grade IV tumors was higher (11.8 ± 2.45%; range: 8.14–15.33%) compared to grade III (7.42 ± 0.31%; range: 7.2–7.65%) or grade II (2.33 ± 0.12%; range: 2.25–2.42%) (P < 0.05). Total absence of HO-1 expression was noted in healthy brain and sorted tumor cells. GAPDH expression was used to normalize gene expression. Relative expression was calculated for HO-1 gene by using ΔCT method. The data shown is the mean ± SD
Induction of HO-1 expression in CD4+CD25+FoxP3+ T-cells is dependent on tumor grade
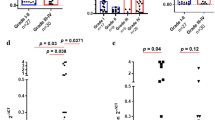
To further confirm that HO-1 was expressed only by glioma infiltrating Treg, we performed a FACS analysis of total CD4+ T-cells and CD4+CD25+FoxP3+ after gating in total CD3+ T-cells, using anti-HO-1 mAb. The frequency of HO-1 expression was analyzed in various tumor grades. The results, shown in Fig. 4A, illustrate progressive HO-1 expression in glioma infiltrating total CD4+ T-cells. The percentage of HO-1 expressing Treg was 31.1 ± 2.2% in grade IV tumors as compared to 12 ± 1.7% grade III tumors, and 7.78 ± 1.1% grade II tumors (P < 0.02). Furthermore, the induction of HO-1 expression was highest in CD4+CD25+FoxP3+ subset purified from patients with grade IV glioma (92.3 ± 1.75%) as compared to grade III (70.3 ± 2.3%) or grade II tumors (56.4 ± 2.86%) (P < 0.02) (Fig. 4B).
FACS analysis of HO-1 expression in Treg. (A) Total CD4+ T-cells were gated electronically on the TIL and analyzed versus HO-1 in two-color. (B) Gated CD4+CD25+ or CD4+CD25− were then analyzed versus HO-1 in tricolor. As shown in the figure, there was selective induction of HO-1 expression in CD4+CD25+ (red plot represents isotype control; blue is specific staining). Specifically, the induction of HO-1 expression was highest in CD4+CD25+FoxP3+ subset purified from patients with grade IV glioma (92.3 ± 1.75%) as compared to grade III (70.3 ± 2.3%) or grade II tumors (56.4 ± 2.86%) (P < 0.02) (Fig. 4B). In contrast, there was no evidence of HO-1 expression in CD4+CD25− (data not shown)
The level of HO-1 directly correlates with FoxP3 expression
The expression of HO-1 was correlated with CD4+CD25+FoxP3+ infiltration. The coefficient of correlation between HO-1 mRNA and Foxp3 mRNA was 0.966 (Fig. 5). This result confirms that HO-1 mRNA expression correlates with the induction of Foxp3 in CD4+CD25+ glioma infiltrating Treg.
Discussion
Heme oxygenase-1, a key enzyme in heme catabolism, has recently been reported to be expressed by human brain tumors [23], to represent a useful marker for angiogenesis in human gliomas [24], and to accumulate during oligodendroglioma progression [25]. To date, only macrophages/microglia present in the stromal compartment of tumors have been shown to express HO-1 [24, 25]. In this study, we provide the first report to show that the expression of HO-1 and FoxP3 directly correlate with tumor grade. Moreover, we show that the expression of HO-1 is not unique to gliomas but is rather a function of Treg infiltration, and the increased infiltration of high-grade tumors with Treg is an important determinant of the immunosuppressive environment exhibited by malignant brain tumors. These are significant findings, which elucidate the mechanism of HO-1 induction and expression in human gliomas.
HO-1 has an important role in rapid tumor growth because of its antioxidative and antiapoptotic effects [29, 30]. Indeed, there is convincing evidence that accumulation of HO-1 expressing macrophages in necrotic areas of malignant brain tumors contributes to neoplastic growth and tissue damage [24]. The expression of HO-1 is activated in response to various environmental insults, including hypoxia, heat shock, heavy metal toxicity, and UV light irradiation [31, 32]. Although the accumulation of HO-1 expressing Treg may be induced by irradiation and chemotherapy administered to the patients (Table 1), our published findings of Treg infiltration even in newly diagnosed gliomas [11] would argue against this hypothesis. Indeed, even in the present study we did not detect any significant difference in Treg infiltration between newly diagnosed versus recurrent grade IV astrocytomas. As such, we propose that Treg progressively infiltrate gliomas at various stages of the disease. In turn, this leads to localized HO-1 expression. The expression of HO-1 by Treg along with local tissue hypoxia/re-oxygenation stimulates a number of cytokines, proteolytic enzymes, and inhibitors, which play key role in the angiogenesis cascade. Indeed, HO-1 mediated heme degradation is the primary mechanism for cellular carbon monoxide (CO) production, and reactive oxygen intermediates, including CO, induce angiogenesis in vitro and in vivo [33]. Most recent studies confirm that HO-1 is a useful marker for neovascularization of human gliomas [24].
The exact role of HO-1 expression is not fully understood in T-cells. However, growing evidence has shown that HO-1 and its byproduct, CO, suppress T-cell proliferation [34, 35]. The over-expression of HO-1 results in the inhibition of several immune effector functions and thus provides an explanation for stress-induced immunosuppression [36]. Moreover, human CD4+CD25+ T-cell constitutively express HO-1 [26]. It would appear the FoxP3 induces HO-1 expression and the suppressive effect of Foxp3 is not observed in the presence of HO-1 siRNA [28]. These findings provide a compelling argument that human FoxP3 may be a crucial regulatory gene for the expression of HO-1 and that HO-1 is engaged in FoxP3 mediated immune suppression.
In conclusion, we have shown that (1) Treg progressively infiltrate gliomas with increasing tumor grade; and (2) the expression of HO-1 directly correlates with the expression of Foxp3, a marker of Treg. Although we are aware that the small number of patients with grade II and grade III tumor may limit these conclusions, the data provides a compelling argument for a more extensive investigation of HO-1 and FoxP3 expression in a larger number of patients. Our findings suggest that, at least in part, the expression of HO-1 by gliomas is dependent on the presence of CD4+CD25+ regulatory T-cells. Selective targeting of either HO-1 or FoxP3 may therefore have important therapeutic implications in the setting of malignant gliomas.
References
Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ (2005) Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol 54:369–377
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108:804–811
Chattopadhyay S, Chakraborty NG, Mukherji B (2005) Regulatory T cells and tumor immunity. Cancer Immunol Immunother 54:1153–1161
Kohno T, Yamada Y, Akamatsu N, Kamihira S, Imaizumi Y, Tomonaga M, Matsuyama T (2005) Possible origin of adult T-cell leukemia/lymphoma cells from human T lymphotropic virus type-1-infected regulatory T cells. Cancer Sci 96:527–533
Linehan DC, Goedegebuure PS (2005) CD25+CD4+ regulatory T-cells in cancer. Immunol Res 32:155–168
Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL (2005) Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 92:913–920
Wang RF (2006) Functional control of regulatory T cells and cancer immunotherapy. Semin Cancer Biol 16:106–114
Wang RF (2006) Immune suppression by tumor-specific CD4+ regulatory T-cells in cancer. Semin Cancer Biol 16:73–79
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Wolf D, Rumpold H, Wolf AM (2006) Regulatory T cells in cancer biology: a possible new target for biochemical therapies. Mini Rev Med Chem 6:509–513
El Andaloussi A, Lesniak MS (2006) An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-oncol 8(3):234–243
El Andaloussi A, Han Y, Lesniak MS (2006) Prolongation of survival following depeletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg 105(3):430–437
Maines M (1998) Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. Faseb J 2:2557–2568
Stocker R, Glazer AN, Ames BN (1987) Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA 84:5918–5922
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046
Trakshel GM, Maines MD (1989) Multiplicity of heme oxygenase isozymes. HO-1 and HO-2 are different molecular species in rat and rabbit. J Biol Chem 264:1323–1328
Willis D, Moore AR, Willoughby DA (2000) Heme oxygenase isoform expression in cellular and antibody-mediated models of acute inflammation in the rat. J Pathol 190:627–634
Ewing JF, Haber SN, Maines MD (1992) Normal and heat-induced patterns of expression of heme oxygenase-1 (HSP32) in rat brain: hyperthermia causes rapid induction of mRNA and protein. J Neurochem 58:1140–1149
Panahian N, Yoshiura M, Maines MD (1999) Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem 72:1187–1203
Fukuda K, Panter SS, Sharp FR, Noble LJ (1995) Induction of heme oxygenase-1 (HO-1) after traumatic brain injury in the rat. Neurosci Lett 199:127–130
Nimura T, Weinstein PR, Massa SM, Panter S, Sharp FR (1996) Heme oxygenase-1 (HO-1) protein induction in rat brain following focal ischemia. Brain Res Mol Brain Res 37:201–208
Schipper HM, Cisse S, Stopa EG (1995) Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann Neurol 37:758–768
Hara E, Takahashi K, Tominaga T, Kumabe T, Kayama T, Suzuki H, Fujita H, Yoshimoto T, Shirato K, Shibahara S (1996) Expression of heme oxygenase and inducible nitric oxide synthase mRNA in human brain tumors. Biochem Biophys Res Commun 224:153–158
Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M (1999) Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 5:1107–1113
Deininger MH, Meyermann R, Trautmann K, Duffner F, Grote EH, Wickboldt J, Schluesener HJ (2000) Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res 882:1–8
Pae HO, Oh GS, Choi BM, Chae SC, Chung HT (2003) Differential expressions of heme oxygenase-1 gene in CD25- and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun 306:701–705
Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, Kim YM, Chung HT (2004) Overexpression of heme oxygenase (HO)-1 renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radic Biol Med 36:858–871
Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT (2005) Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun 327:1066–1071
Doi K, Akaike T, Fujii S, Tanaka S, Ikebe N, Beppu T, Shibahara S, Ogawa M, Maeda H (1999) Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer 80:1945–1954
Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, Miyamoto Y, Maeda H (2003) Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer 88:902–909
Dwyer BE, Nishimura RN, Lu SY, Alcaraz A (1996) Transient induction of heme oxygenase after cortical stab wound injury. Brain Res Mol Brain Res 38:251–259
Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM (1997) Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272:5375–5381
Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP (1996) Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest 98:1667–1675
Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT (2004) Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol 172:4744–4751
Song R, Mahidhara RS, Zhou Z, Hoffman RA, Seol DW, Flavell RA, Billiar TR, Otterbein LE, Choi AM (2004) Carbon monoxide inhibits T lymphocyte proliferation via caspase-dependent pathway. J Immunol 172:1220–1226
Woo J, Iyer S, Cornejo MC, Mori N, Gao L, Sipos I, Maines M, Buelow R (1998) Stress protein-induced immunosuppression: inhibition of cellular immune effector functions following overexpression of haem oxygenase (HSP 32). Transpl Immunol 6:84–93
Acknowledgements
This work was supported by the University of Chicago Cancer Research Center, The Brain Research Foundation, and The Ibrahim Bodur Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
El Andaloussi, A., Lesniak, M.S. CD4+CD25+FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol 83, 145–152 (2007). https://doi.org/10.1007/s11060-006-9314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9314-y