Abstract
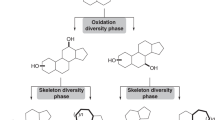
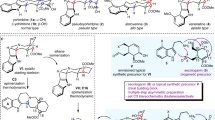
The solid-phase synthesis of 16α-derivatives of 5α-androstane-3α,17β-diol with one, two or three levels of molecular diversity was accomplished using the diethylsilyloxy linker. Libraries with one level of diversity (10 members) and two levels of diversity (40 members) were synthesized in a parallel fashion in good yields and acceptable HPLC purities for the majority of library members. Compounds with three levels of diversity (15 pools) were realized in a split and pool fashion to allow further deconvolution by the positional scanning method. The screening of the generated model libraries revealed interesting preliminary structure–activity relationships related to their antiproliferative activities on androgen-sensitive Shionogi cells. In the case of the two-level library, the presence of a hydrophobic amino acid at R1 (isoleucine (Ile) or phenylalanine (Phe)) and a six-membered ring (aromatic or not) at R2 seems an important requirement for activity. In the three-level library, the amino acid residues isoleucine and phenylalanine clearly provided a better antiproliferative activity than glycine (Gly) and proline (Pro). These model libraries will serve as basis for the generation of larger libraries of peptidosteroids toward the development of therapeutic agents.
Similar content being viewed by others
Abbreviations
- ADT:
-
androsterone
- AR:
-
androgen receptor
- DHP:
-
dihydropyran
- DHT:
-
dihydrotestosterone
- 3α-diol:
-
5α-androstane-3α,17β-diol
- DIPEA:
-
diisopropylethylamine
- DMF:
-
dimethylformamide
- DMSO:
-
dimethylsulfoxide
- EtOAc:
-
ethyl acetate
- Fmoc:
-
9-fluorenylmethoxycarbonyl
- Gly:
-
glycine
- h:
-
hour
- HMPA:
-
hexamethylphosphoramide
- HOBt:
-
N-hydroxybenzotriazole
- HPLC:
-
high-performance liquid chromatography
- HSD:
-
hydroxysteroid dehydrogenase
- IR:
-
infrared spectroscopy
- Ile:
-
isoleucine
- LCMS:
-
liquid chromatography mass spectrometry
- LDA:
-
lithium diisopropylamide
- LRMS:
-
low resolution mass spectrometry
- MS:
-
mass spectrometry
- MeOH:
-
methanol
- min:
-
minute
- NMR:
-
nuclear magnetic resonance
- Ph:
-
phenyl
- Phe:
-
phenylalanine
- Pro:
-
proline
- PS-DES:
-
polystyrene diethylsilyl resin
- p-TSA:
-
p-toluenesulfonic acid
- PyBOP:
-
benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate
- rt:
-
room temperature
- TBAF:
-
tetrabutylammonium fluoride
- TBDMS:
-
tert-butyldimethylsilyl
- THF:
-
tetrahydrofuran
- THP:
-
tetrahydropyran
- TLC:
-
thin-layer chromatography.
References
Hanson, J.R., Steroids: Reactions and partial synthesis, Nat. Prod. Rep., 19 (2002) 381–389, and references therein.
Maltais, R., Tremblay, M.R., Ciobanu, L. and Poirier, D., Steroids and combinatorial chemistry, J. Comb. Chem., 6 (2004), 443–456.
Poirier, D., Inhibitors of 17β-hydroxysteroid dehydrogenases, Curr. Med. Chem., 10 (2003) 453–477.
Singh, S.M., Gauthier, S. and Labrie, F., Androgen receptor antagonist (antiandrogens): Structure–activity relationships, Curr. Med. Chem., 7 (2000) 211–247.
Newman, D.J., Cragg, G.M. and Snader, K.M., The influence of natural products upon drug discovery, Nat. Prod. Rep., 17 (2000) 215–234.
Bratoeff, E., Ramirez, E., Murillo, E., Flores, G. and Cabeza, M., Steroidal antiandrogens and 5α -reductase inhibitors, Curr. Med. Chem., 6 (1999) 1107–1123.
Poirier, D., Ciobanu, L.C. and Maltais, R., Steroid sulfatase inhibitors, Exp. Opin. Ther. Patents., 9 (1999) 1083–1099.
Hamada, H., Newmann, F. and Junkman, K., Intrauterine antimaskuline beeinflussing von rattenfetendurch ein stark gestagen wirksames steroid, Steroid Acta Endocrinol., 44 (1963) 330.
Labrie, F., Cusan, L., Dupont, A., Gomez, J.L., Simard, J., Luu-The, V., Pelletier, G., Labrie, C. and Bélanger, A., In Reproductive Endocrinology, Surgery and Technology. Lippincott-Raven, Philadelphia, 1996.
Brooks, J.R., Berman, C., Primka, R.L., Reynolds, G.F. and Rasmusson, G.H., 5 Alpha-reductase inhibitory and anti-androgenic activities of some 4-azasteroids in the rat, Steroids, 47 (1986) 1–19.
Tchédam-Ngatcha, B., Luu-The, V. and Poirier, D., Androsterone 3β -substituted derivatives as inhibitors of type 3 17β -hydroxysteroid dehydrogenase, Bioorg. Med. Chem. Lett., 10 (2000) 2533–2536.
Tchédam-Ngatcha, B., Luu-The, V. and Poirier, D., Androsterone derivatives substituted at position 16: Chemical synthesis, inhibition of type 3 17β -hydroxysteroid dehydrogenase, binding affinity for steroid receptors and proliferative/antiproliferative activity on Shionogi (AR+) cells, J. Enz. Inh. Med. Chem., 17 (2002) 155–165.
Fevig, T.L. and Katzenellenbogen, J.A., A short, stereoselective route to 16α-(substituted-alkyl)-estradiol derivatives, J. Org. Chem., 52 (1987) 247–251.
Tremblay, M.R., Auger, S. and Poirier, D., Synthesis of 16-(bromoalkyl)-estradiols having inhibitory effect on human placental estradiol 17β -hydroxysteroid dehydrogenase (17β -HSD type 1), Bioorg. Med. Chem., 3 (1995) 505–523.
Tremblay, M.R. and Poirier, D., Overview of a rational approach to design type I 17β -hydroxysteroid dehydrogenase inhibitors without estrogenic activity: Chemical synthesis and biological evaluation, J. Steroid Biochem. Mol. Biol., 66 (1998) 179–191.
Maltais, R., Tremblay, M.R. and Poirier, D., Solid phase synthesis of hydroxysteroids using the diethylsilyloxy linker, J. Comb. Chem., 2 (2000) 604–614.
Tremblay, M.R and Poirier, D., Solid-phase of phenolic steroids: From optimization studies to a convenient procedure for combinatorial synthesis of biologically relevant estradiol derivatives, J. Comb. Chem., 2 (2000) 48–65.
Hu, Y., Porco, J.A., Labadie, J.W. and Gooding, O.W., Novel polymer-supported trialkylsilanes and their use in solid-phase organic synthesis, J. Org. Chem., 63 (1998) 4518–4521.
Bartra, M., Romea, P., Urpi, F. and Vilarrasa, J., A fast procedure for the reduction of azides and nitro compounds based on the reducing ability of Sn(SR)3-species. Tetrahedron, 46 (1990) 587–594.
Furka, A., Sebestyn, F. and Asgedom, M., General method for rapid synthesis of multicomponent peptide mixtures, Int. J. Pept. Protein Res., 37 (1991) 487–493.
Smith, P.W., Lai, J.Y.Q., Whittington, A.R., Cox, B., Houston, J.G., Stylli, C.H., Banks, M.N. and Tiller, P.R., Synthesis and biological evaluation of a library containing potentially 1600 amides/esters. A strategy for rapid compound generation and screening, Bioorg. Med. Chem. Lett., 4 (1994) 2821–2824.
Pirrung, M.C. and Chen, J., Preparation and screening against acetylcholinesterase of a non-peptide ‘indexed’ combinatorial library, J. Am. Chem. Soc., 117 (1995) 1240–1245.
Sack, J.S., Kish, K.F., Wang, C., Attar, R.M., Kiefer, S.E., An, Y., Wu, G.Y., Scheffler, J.E., Salvati, M.E., Krystek, S.R. Jr., Weinmann, R. and Einspahr, H.M., Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone, Proc. Natl. Acad. Sci. U.S.A., 98 (2001) 4904–4909.
Matias, P.M., Donner, P., Coelho, R., Thomaz, M., Peixoto, C., Macedo, S., Otto, N., Joschko, S., Scholz, P., Wegg, A., Bäsler, S., Schäfer, M., Egner, U. and Carrondo, M.A., Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations, J. Biol. Chem., 275 (2000) 26164–26171.
Luthy, I., Begin, D. and Labrie, F., Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture, J. Steroid Biochem., 31 (1988) 845–852.
Plante, M., Lapointe, S. and Labrie, F., Stimulatory effect of synthetic progestins currently used for the treatment of prostate cancer on growth of the androgen-sensitive Shionogi tumor in mice, J. Steroid Biochem., 31 (1988) 61–64.
Simard, J., Dauvois, S., Haagensen, D.E., Lévesque, C., Mérand, Y. and Labrie, F., Regulation of progesterone binding breast cyst protein GCDFP-24 secretion by estrogens and androgens in human breast cancer cell: a new marker of steroid action in breast cancer, Endocrinology, 126 (1990) 3223–3231.
Fiszer-Szafarz, B., Szafarz, D. and Guevara de Murillo, A., A general, fast and sensitive micromethod for DNA determination: Application to rat and mouse liver, rat hepatoma, human leukocytes, chicken fibroblasts and yeast cells, Anal. Biochem., 110 (1981) 165–170.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maltais, R., Mercier, C., Labrie, F. et al. Solid-phase synthesis of model libraries of 3 α17β-dihydroxy-16α-(aminoethyl-N-substituted)-5α-androstanes for the development of steroidal therapeutic agents. Mol Divers 9, 67–79 (2005). https://doi.org/10.1007/s11030-005-1312-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11030-005-1312-z