Abstract
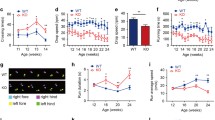
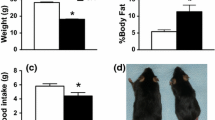
Iron dyshomeostasis has been implicated in many diseases, including a number of neurological conditions. Cytosolic NADH cytochrome b5 oxidoreductase (NCB5OR) is ubiquitously expressed in animal tissues and is capable of reducing ferric iron in vitro. We previously reported that global gene ablation of NCB5OR resulted in early-onset diabetes and altered iron homeostasis in mice. To further investigate the specific effects of NCB5OR deficiency on neural tissue without contributions from known phenotypes, we generated a conditional knockout (CKO) mouse that lacks NCB5OR only in the cerebellum and midbrain. Assessment of molecular markers in the cerebellum of CKO mice revealed changes in pathways associated with cellular and mitochondrial iron homeostasis. 59Fe pulse-feeding experiments revealed cerebellum-specific increased or decreased uptake of iron by 7 and 16 weeks of age, respectively. Additionally, we characterized behavioral changes associated with loss of NCB5OR in the cerebellum and midbrain in the context of dietary iron deprivation-evoked generalized iron deficiency. Locomotor activity was reduced and complex motor task execution was altered in CKO mice treated with an iron deficient diet. A sucrose preference test revealed that the reward response was intact in CKO mice, but that iron deficient diet consumption altered sucrose preference in all mice. Detailed gait analysis revealed locomotor changes in CKO mice associated with dysfunctional proprioception and locomotor activation independent of dietary iron deficiency. Finally, we demonstrate that loss of NCB5OR in the cerebellum and midbrain exacerbated harmaline-induced tremor activity. Our findings suggest an essential role for NCB5OR in maintaining both iron homeostasis and the proper functioning of various locomotor pathways in the mouse cerebellum and midbrain.







Similar content being viewed by others
Notes
Wang WF, et al., NCB5OR deficiency causes anemia, iron dyshomeostasis and increased susceptibility to mitochondrial dysfunction. Manuscript in preparation;
Wang WF, et al., Monogenic NCB5OR diabetes is a result of impaired iron homeostasis and mitochondrial dysfunction in beta-cells. Manuscript in preparation;
Low-Fe is used in lieu of low-iron for figures.
Stroh MA, et al., NCB5OR deficiency in the cerebellum and midbrain affects fasted feeding behavior, thirst response, and voluntary exercise in mice. Publication in process.
Abbreviations
- NCB5OR:
-
NADH Cytochrome b5 Oxidoreductase
- CKO:
-
Conditional knockout
- WT:
-
Wild-type
- MPP:
-
Motion power percentage
- VTA:
-
Ventral tegmental area
References
Andersen G et al (2004) Variation in NCB5OR: studies of relationships to type 2 diabetes, maturity-onset diabetes of the young, and gestational diabetes mellitus. Diabetes 53(11):2992–2997
Belaidi AA, Bush AI (2015) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem. doi:10.1111/jnc.13425
Bourdy R et al (2014) Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacol 39(12):2788–2798
Bourque SL, Iqbal U, Reynolds JN, Adams MA, Nakatsu K (2008) Perinatal iron deficiency affects locomotor behavior and water maze performance in adult male and female rats. J Nutr 138(5):931–937
Brehm MA, Powers AC, Shultz LD, Greiner DL (2012) Advancing animal models of human type 1 diabetes by engraftment of functional human tissues in immunodeficient mice. Cold Spring Harb Perspect Med 2(5):a007757
Capoccia S et al (2015) Behavioral characterization of mouse models of neuroferritinopathy. PloS one 10(2):e0118990
Cho SS, Shin DH, Lee KH, Hwang DH, Chang KY (1998) Localization of transferrin binding protein in relation to iron, ferritin, and transferrin receptors in the chicken cerebellum. Brain Res 794(1):174–178
Connor JR et al (2003) Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 61(3):304–309
de Jong JW et al (2015) Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation. Neuropsychopharmacol 40(9):2085–2095
Du X et al (2008) The serine protease TMPRSS6 is required to sense iron deficiency. Science 320(5879):1088–1092
Duce JA et al (2010) Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142(6):857–867
Earley CJ, Connor JR, Beard JL, Clardy SL, Allen RP (2005) Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep 28(9):1069–1075
Faber S, Zinn GM, Kern JC, Kingston HMS (2009) The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 14(3):171–180
Fernandez-Real JM, McClain D, Manco M (2015) Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 38(11):2169–2176
Fiset C, Rioux FM, Surette ME, Fiset S (2015) Prenatal iron deficiency in guinea pigs increases locomotor activity but does not influence learning and memory. PloS one 10(7):e0133168
Guo B, Yu Y, Leibold EA (1994) Iron regulates cytoplasmic levels of a novel iron-responsive element-binding protein without aconitase activity. J Biol Chem 269(39):24252–24260
Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS (2008) Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry 65(10):1136–1144
Iwai K, Klausner RD, Rouault TA (1995) Requirements for iron-regulated degradation of the RNA binding protein, iron regulatory protein 2. EMBO J 14(21):5350–5357
Kalman FS et al (2013) Natural mutations lead to enhanced proteasomal degradation of human Ncb5or, a novel flavoheme reductase. Biochimie 95(7):1403–1410
Kim JY et al (2014) Sequential accumulation of iron in glial cells during chicken cerebellar development. Acta Histochem 116(4):570–576
Kimmel RA et al (2000) Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev 14(11):1377–1389
Koirala S, Corfas G (2010) Identification of novel glial genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum. PLoS One 5(2), e9198
Konofal E, Lecendreux M, Arnulf I, Mouren MC (2004) Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 158(12):1113–1115
Konofal E et al (2007) Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med 8(7–8):711–715
Konofal E et al (2008) Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol 38(1):20–26
Larade K et al (2008) Loss of Ncb5or results in impaired fatty acid desaturation, lipoatrophy, and diabetes. J Biol Chem 283(43):29285–29291
LaVaute T et al (2001) Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27(2):209–214
Lemaire-Vieille C et al (2013) Ataxia with cerebellar lesions in mice expressing chimeric PrP-Dpl protein. J Neurosci 33(4):1391–1399
Maccarinelli F et al (2015) A novel neuroferritinopathy mouse model (FTL 498InsTC) shows progressive brain iron dysregulation, morphological signs of early neurodegeneration and motor coordination deficits. Neurobiol Dis 81:119–133
Martin FC, Le AT, Handforth A (2005) Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord 20(3):298–305
Miyajima H et al (2001) Cerebellar ataxia associated with heteroallelic ceruloplasmin gene mutation. Neurology 57(12):2205–2210
Moos T, Mollgard K (1993) A sensitive post-DAB enhancement technique for demonstration of iron in the central nervous system. Histochemistry 99(6):471–475
Mori S et al (1998) Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann N Y Acad Sci 860:94–105
Nguyenlegros J, Bizot J, Bolesse M, Pulicani JP (1980) Diaminobenzidine black as a new histochemical-demonstration of exogenous iron. Histochemistry 66(3):239–244
Olney DK et al (2007) Young Zanzibari children with iron deficiency, iron deficiency anemia, stunting, or malaria have lower motor activity scores and spend less time in locomotion. J Nutr 137(12):2756–2762
Percinel I, Yazici KU, Ustundag B (2015) Iron deficiency parameters in children and adolescents with attention-deficit/hyperactivity disorder. Child Psychiatry Hum Dev 47(2):259–269
Provini F, Chiaro G (2015) Neuroimaging in restless legs syndrome. Sleep Med Clin 10(3):215–226
Qu S et al (2007) Locomotion is increased in A11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol 66(5):383–388
Rasouli J, Lekhraj R, Ozbalik M, Lalezari P, Casper D (2011) Brain-spleen inflammatory coupling: a literature review. Einstein J Biol Med 27(2):74–77
Rogers JT et al (2002) An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 277(47):45518–45528
Roschzttardtz H, Conejero G, Curie C, Mari S (2010) Straightforward histochemical staining of Fe by the adaptation of an old-school technique: identification of the endodermal vacuole as the site of Fe storage in Arabidopsis embryos. Plant Signal Behav 5(1):56–57
Russo AJ (2009) Anti-metallothionein IgG and levels of metallothionein in autistic children with GI disease. Drug Healthcare Patient Saf 1:1–8
Salamone JD (1992) Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology 107(2–3):160–174
Samaniego F, Chin J, Iwai K, Rouault TA, Klausner RD (1994) Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. Structure, function, and post-translational regulation. J Biol Chem 269(49):30904–30910
Smith MA, Harris PLR, Sayre LM, Perry G (1997) Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 94(18):9866–9868
Solbach K et al (2014) Cerebellar pathology in Friedreich’s ataxia: atrophied dentate nuclei with normal iron content. Neuroimage Clin 6:93–99
Stroh M, Swerdlow RH, Zhu H (2014) Common defects of mitochondria and iron in neurodegeneration and diabetes (MIND): a paradigm worth exploring. Biochem Pharmacol 88(4):573–583
Thomas DG, Grant SL, Aubuchon-Endsley NL (2009) The role of iron in neurocognitive development. Dev Neuropsychol 34(2):196–222
Torrance JD, Bothwell TH (1968) A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci 33(1):9–11
Unger EL, Sterling ME, Jones BC, Beard JL (2008) Genetic variations in ventral midbrain iron influence diurnal locomotor activity and body temperature in BXD mice. FASEB J 22:694.10
Vela G et al (2015) Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast 2015:972791
Wang WF et al (2011) Development of diabetes in lean Ncb5or-null mice is associated with manifestations of endoplasmic reticulum and oxidative stress in beta cells. BBA Mol Basis Dis 1812(11):1532–1541
WHO (2001) Iron deficiency anaemia: assessment, prevention and control
Xie J et al (2004) Absence of a reductase, NCB5OR, causes insulin-deficient diabetes. Proc Natl Acad Sci U S A 101(29):10750–10755
Xie Y et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379
Xu M et al (2011) Ncb5or deficiency increases fatty acid catabolism and oxidative stress. J Biol Chem 286(13):11141–11154
Ye H, Rouault TA (2010) Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry 49(24):4945–56
Zhao L, Hadziahmetovic M, Wang C, Xu X, Song Y, Jinnah HA, Wodzinska J, Iacovelli J, Wolkow N, Krajacic P, Weissberger AC, Connelly J, Spino M, Lee MK, Connor J, Giasson B, Leah Harris Z, Dunaief JL (2015) Cp/Heph mutant mice have iron-induced neurodegeneration diminished by deferiprone. J Neurochem 135(5):958–974
Zhu H, Qiu H, Yoon HW, Huang S, Bunn HF (1999) Identification of a cytochrome b-type NAD(P)H oxidoreductase ubiquitously expressed in human cells. Proc Natl Acad Sci U S A 96(26):14742–14747
Zhu H et al (2004) NCB5OR is a novel soluble NAD(P)H reductase localized in the endoplasmic reticulum. J Biol Chem 279(29):30316–30325
Zhu H, Wang WF, Wang HP (2013) Impaired iron metabolism in monogenic Ncb5or diabetes. Diabetes 62:A566–A566
Zucca FA et al (2015) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol. doi:10.1016/j.pneurobio.2015.09.012
Acknowledgments
Authors thank Dr. John Stanford (University of Kansas Medical Center, KUMC) for providing critiques and comments for the manuscript. Authors acknowledge Dr. WenFang Wang (KUMC) for preparing the NCB5OR-floxed line for crossing and Dr. Alexandra Joyner at Memorial Sloan-Kettering Cancer Center for providing the En1-cre driver. This study was supported by the KUMC School of Health Professions research funds (H.Z.) and a Ruth L. Kirschstein National Research Service Award (T32 HD057850, PI: R. Nudo) supporting M.A.S. Access to the KUMC Rodent Behavior Facility and shared equipment core facilities was provided by the Kansas Intellectual and Developmental Disabilities Research Center (P30 HD02528).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Rights and permissions
About this article
Cite this article
Stroh, M.A., Winter, M.K., Swerdlow, R.H. et al. Loss of NCB5OR in the cerebellum disturbs iron pathways, potentiates behavioral abnormalities, and exacerbates harmaline-induced tremor in mice. Metab Brain Dis 31, 951–964 (2016). https://doi.org/10.1007/s11011-016-9834-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-016-9834-x