Abstract
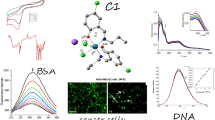
Cisplatin and other metal-based drugs often display side effects and tumor resistance after prolonged use. Because rhenium-based anticancer complexes are often less toxic, a novel series of organorhenium complexes were synthesized of the types: XRe(CO)3Z (X = α-diimines and Z = p-toluenesulfonate, 1-naphthalenesulfonate, 2-naphthalenesulfonate, picolinate, nicotinate, aspirinate, naproxenate, flufenamate, ibuprofenate, mefenamate, tolfenamate, N-acetyl-tryptophanate), and their biological properties were examined. Specifically, in hormone-dependent MCF-7 and hormone-independent triple-negative MDA-MB-231 breast cancer cells, the p-toluenesulfonato, 1-naphthalenesulfonato, 2-naphthalenesulfonato, picolinato, nicotinato, acetylsalicylato, flufenamato, ibuprofenato, mefenamato, and N-acetyl-tryptophanato complexes were found to be far more potent than conventional drug cisplatin. DNA-binding studies were performed in each case via UV–Vis titrations, cyclic voltammetry, gel electrophoresis, and viscosity, which suggest DNA partial intercalation interaction, and the structure–activity relationship studies suggest that the anticancer activities increase with the increasing lipophilicities of the compounds, roughly consistent with their DNA-binding activities.








Similar content being viewed by others
References
Wrighton M, Morse DL (1974) The nature of the lowest excited state in tricarbonyl-chloro-1,10-phenanthrolinerhenium(I) and related complexes. J Am Chem Soc 96(4):998–1003
Sacksteder L, Lee M, Demas JN, DeGraff BA (1993) Long-lived, highly luminescent rhenium(I) complexes as molecular probes: intra and intermolecular excited-state interactions. J A Chem Soc. 115:8230–8238
Yam VWW, Lau VCY, Cheung KK (1995) Synthesis, photophysics and photo-chemistry of novel luminescent rhenium (I) photoswitchable materials. J Chem Soc Chem Commun 2:259–261
Ehler TT, Malmberg N, Carron K, Sullivan BP, Noe LJ (1997) Studies of organometallic self-assembled monolayers on Ag and Au using surface Plasmon spectroscopy. J Phys Chem B. 101:3174–3180
Kotch TG, Lees AJ, Fuerniss SJ, Papathomas K, Snyder IRW (1993) Luminescence rigidochromism of fac-tricarbonylchloro(4,7-diphenyl-1,10-phenanthroline)rhenium as a spectroscopic robe in monitoring polymerization of photosensitive thin films. Inorg Chem 32:2570–2575
Stoeffler HD, Thornton NB, Temkin SL, Schanze KS (1995) Unusual photophysics of a rhenium(I) dipyridophenazine complex in homogeneous solution and bound to DNA. J Am Chem Soc 117:7119–7128
Oriskovich TA, White PS, Thorp HH (1995) Luminescent labels for purine nucleobases: electronic properties of guanine bound to rhenium(I). Inorg Chem 34:1629–1631
Yan YK, Cho SE, Shaffer KA, Rowell JE, Barnes BJ, Hall IH (2000) Cytotoxicity of rhenium(I) alkoxo and hydroxo carbonyl complexes in murine and human tumor cells. Pharmazie 55:307–313
Leonidova A, Gasser G (2014) Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem Biol 9:2180–2193
Mbagu MK, Kebulu DN, Winstead A, Pramanik SK, Banerjee HN, Iwunze MO, Wachira JM, Greco GE, Haynes GK, Sehmer A, Sarkar FH, Ho DM, Pike RD, Mandal SK (2012) Fac-Tricarbonyl(pentylcarbonato)(α-diimine)rhenium complexes: one-pot synthesis, characterization, fluorescence studies, and cytotoxic activity against human MDA-MB-231 breast, CCl-227 colon and BxPC-3 pancreatic carcinoma cell lines. Inorg Chem Commun 21:35–38
The synthetic procedure has been presented at the American Chemical Society National Meetings and can be accessed through the Technical Programming Archive: Past National Meetings (2004-present) and the link is: http://www.acs.org/content/acs/en/meetings/nationalmeetings/programarchive.html?_ga=1.29689146.464482569.1442729062
Parson C, Smith V, Krauss C, Banerjee HN, Reilly C, Krause JA, Wachira JM, Giri D, Winstead A, Mandal SK (2013) The effect of novel rhenium compounds on lymphosarcoma, PC-3 prostate and myeloid leukemia cancer cell lines and an Investigation on the DNA binding properties of one of these compounds through electronic spectroscopy. J Bioproces Biotechniq. 4:1–5
Wang W, Yan YK, Andy Hor TS, Vittal JJ, Wheaton JR, Hall IH (2002) Synthesis, X-ray structures, and cytotoxicity of rhenium(I) carbonyl 2-(dimethylamino)-ethoxide complexes. Polyhedron 21:1991–1999
Kumar CA, Carthikeyan S, Varghese B, Veena V, Sakthivel N, Manimaran B (2014) Synthesis, characterization and cytotoxicity evaluation of rhenium(I) based ester functionalized dinuclear metallacyclophanes. J Organomet Chem 766:86–94
Ye R-R, Tan C-P, Chen MH, Hao L, Ji LN, Mao Z-W (2016) Mono- and dinuclear phosphorescent rhenium(I) complexes: impact of subcellular localization on anticancer mechanisms. Chem Eur J 22:7800–7809
Dallagi T, Saidi M, Vessieres A, Huche M, Jaouen G, Top S (2013) Synthesis and antiproliferative evaluation of ferrocenyl and cymantrenyl triaryl butene on breast cancer cells. biodistribution study of the corresponding technetium-99 m tamoxifen conjugate. J Organomet Chem 734:69–77
Cazares-Marinero JJ, Top S, Vessieres A, Jaouen G (2013) Synthesis and antiproliferative activity of hydroxyferrocifen hybrids against triple-negative breast cancer cells. Dalton Trans 43:817–830
Cázares-Marinero JJ, Buriez O, Labbé E, Top S, Amatore C, Jaouen G (2013) Synthesis, characterization, and antiproliferative activities of novel ferrocenophanic suberamides against human triple-negative MDA-MB-231 and hormone-dependent MCF-7 breast cancer cells. Organometallics 32:5926–5934
Medley J, Payne G, Banerjee HN, Giri D, Winstead A, Wachira JM, Krause JA, Shaw R, Pramanik SK, Mandal SK (2015) DNA-binding and cytotoxic efficacy studies of organorhenium pentylcarbonate compound. Mol Cell Biochem 398:21–30
Banerjee HN, Boston A, Barfield A, Stevenson M, Sarkar FH, Giri D, Winstead A, Krause JA, Mandal SK (2016) A study of the effects of novel rhenium compounds on pancreatic and prostate cancer cell lines. Int J Sci Res. 5(7):481–483
Dimitrakopoulou A, Dendrinou-Samara C, Pantazaki AA, Alexiou M, Nordlander E, Kessissoglou DP (2008) Synthesis, structure and interactions with DNA of novel tetranuclear, [Mn4(II/II/II/IV)] mixed valence complexes. J Inorg Biochem 102(4):618–628
Waring MJ (1965) Complex formation between ethidium bromide and nucleic acids. J Mol Biol 13:269–282
Bullock JP, Carter E, Johnson R, Kennedy AT, Key SE, Kraft BJ, Saxon D, Underwood P (2008) Reactivity of electrochemically generated rhenium (II) tricarbonyl α-diimine complexes: a reinvestigation of the oxidation of luminescent Re(CO)3(α-diimine)Cl and related compounds. Inorg Chem 47(17):7880–7887
Kaplanis M, Stamatakis G, Papakonstantinou VD, Paravatou-Petsotas M, Demopoulos CA, Mitsopoulou CA (2014) Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J Inorg Biochem 135:1–9
Ma D-L, Che C-M, Siu F-M, Yang M, Wong K-Y (2007) DNA binding and cytotoxicity of ruthenium(II) and rhenium(I) complexes of 2-amino-4-phenylamino-6-(2-pyridyl)-1,3,5-triazine. Inorg Chem 46:740–749
Mitsopoulou CA, Dagas C (2010) Synthesis, characterization, DNA binding, and photocleavage activity of oxorhenium (V) complexes with α-diimine and quinoxaline ligands. Bioinorg Chem Appl. doi:10.1155/2010/973742
Suntharalingam K, Awuah SG, Bruno PM, Johnstone TC, Wang F, Lin W, Zheng Y-R, Page JE, Hemann MT, Lippard SJ (2015) Necroptosis-inducing rhenium(V) oxo complexes. J Am Chem Soc 137:2967–2974
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, Gralow J, Yeates K, Taylor C, Oomman N, Krishnan S, Sullivan R, Kombe D, Blas MM, Parham G, Kassami N, Conteh L. The global burden of women’s cancers: a grand challenge in global health (www.thelancet.com Published online November 1, 2016). http://dx.doi.org/10.1016/S0140-6736(16)31392-7.
Cancer deaths among women to rise 60% by 2030, new reports warn. The Guardian, November 1, 2016
Acknowledgements
This research was partially supported by the NCI disability supplement, and the NIH Grant No. T34 GM100831 to HNB and the NIH Grant No. G11HD038439; and Nuclear Regulatory Commission Grant No. NRC-HQ-12-G-27-0086 to SKM. The work was also supported, in part, with funds from the Center for Biomolecular Therapeutics (CBT) at the University of Maryland, School of Medicine (from DJW).
Author contributions
The authors’ contributions are as follows: Conceptualization: SKM, SKP, and HNB; Synthesis: TO, SP, TVH, SA, MS, DG, PO, BVP, and AW; Characterizations: AW, TO, and ST; Cytotoxicity studies: PTW and DJW; X-ray structure determinations: CC, JAG, ALR, JAK, DMH and PYZ; Structure optimizations: RS; DNA-binding studies: TO, SP, TVH, SA, MS, DG, BVP, AB, YZ, SG, PM and JMW; Writing of the original manuscript: SKM, SKP, HNB, DJW and PTW; Reviewing–editing: DJW, PTW, SKP, AW, HNB, DMH, ALR, YZ, RS and JMW; Final document approval: DJW and SKM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare in this research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 2869 kb)
S1 Table. Change in fluorescence intensity after 72-h treatment of MCF-7 and MDA-MB-231 breast cancer cells with various concentrations of the organorhenium sulfonato and carboxylate complexes
Supplementary material 2 (DOCX 24 kb)
S2 Table. The IC50 values (in µM) of all organorhenium complexes on MCF-7 breast cancer cells
Supplementary material 3 (DOCX 17 kb)
S3 Table. The IC50 values (in µM) of all organorhenium complexes on MDA-MB-231 breast cancer cells
Supplementary material 4 (DOCX 9894 kb)
S1 – S9 Figs. IR spectra of TOS7, TOS6, NIC7, 1NS7, 2NS6, PIC7, IB6, PIC6 and 1NS6, respectively, in CH 2 Cl 2 . Each spectrum is shown in the C ≡ O and C = O regions, 2200-1600 cm−1
S10 – S27 Figs. 1 H and 13 C NMR spectra of TOS7, TOS6, NIC7, 1NS7, 2NS6, PIC7, IB6, PIC6 and 1NS6, respectively. CD2Cl2 was used for NIC7, 1NS7, 2NS6, IB6, PIC6 and 1NS6 and CDCl3 was used for TOS7, TOS6 and PIC7
Supplementary material 5 (DOCX 198 kb)
S28 - S35 Figs. UV titration graphs. Electronic absorption spectra for the titration of 25 µM of TOS6, 1NS6, 2NS6, NIC7, PIC7, PIC6, and IB6 and 75 µM of 1NS7, respectively, in the absence and presence of varied amount of DNA. The concentration of the stock solution of DNA was 1693 µM. The corresponding Kb graphs are shown in the insets
Supplementary material 6 (DOCX 674 kb)
S36 – S43 Figs. Cyclic voltammograms. TOS7 (0.6 mM), TOS6 (0.7 mM), 1NS7 (0.6 mM), 2NS6 (0.7 mM), NIC7 (0.7 mM), PIC7 (0.9 mM), PIC6 (0.8 mM), IB6 (0.7 mM) in the absence and presence of 0.1 mM DNA. Scan rate 0.05 V/s
Supplementary material 7 (DOCX 273 kb)
S44 Fig. X-ray structures. (a) TOS2, (b) ASP2, and (c) NIC4 showing the planarity of the phenanthroline rings
Supplementary material 8 (DOCX 67 kb)
S45 Fig. Optimized structures. (a) NIC-7, (b) PIC-7, (c) PIC-6 and (d) TOS-6 obtained from DFT calculations showing the planarity of the phenanthroline rings
Rights and permissions
About this article
Cite this article
Wilder, P.T., Weber, D.J., Winstead, A. et al. Unprecedented anticancer activities of organorhenium sulfonato and carboxylato complexes against hormone-dependent MCF-7 and hormone-independent triple-negative MDA-MB-231 breast cancer cells. Mol Cell Biochem 441, 151–163 (2018). https://doi.org/10.1007/s11010-017-3181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3181-z