Abstract
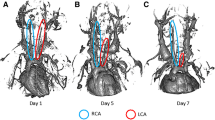
Recent studies suggest that disturbed blood flow-induced shear stress can induce atherosclerosis (ATH) in humans and animals without a high fat diet. Therefore, we hypothesize that partial ligation of the left carotid artery can generate disturbed blood flow and shear stress and would lead to ATH in a predisposed genetic model of Apo E−/− mice. The partial left carotid artery model was generated by ligating three out of four branches of the left carotid artery compared with controls which experienced similar surgery conditions but no ligation. Animals were sacrificed 2 weeks post-ligation and examined for plaque formation, infiltration of leukocytes, pro-inflammatory immune response, and blood flow velocity. Our findings suggest a significant (p < 0.05) increase in plaque formation and lipid deposition in the partial ligated animals compared with controls, confirmed with hematoxylin and eosin and oil red O staining. Furthermore, there was a significant (p < 0.05) increase in the number of M1 macrophages and release of pro-inflammatory cytokines, IL-6 and TNFα, as compared with the control. Moreover, partial ligated carotid arteries demonstrated disturbed blood flow as their systolic velocity was significantly reduced. In conclusion, our data suggest that partial ligation of the left carotid artery induces disturbed flow and shear stress in the predisposed genetic model of Apo E−/− mice and leads to significantly developed ATH. Similarities to clinical patients who develop ATH independent of a high fat diet show that this could be a potential animal model to examine various parameters in ATH.





Similar content being viewed by others
References
Civeira F (2004) Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis 173:55–68
Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, Bonaa KH (1996) Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol 16:984–991
Dedoussis GV (2007) Apolipoprotein polymorphisms and familial hypercholesterolemia. Pharmacogenomics 8:1179–1189
Meyrelles SS, Peotta VA, Pereira TM, Vasquez EC (2011) Endothelial dysfunction in the apolipoprotein E-deficient mouse: insights into the influence of diet, gender and aging. Lipids Health Dis 10:211
Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB Jr (1986) Familial apolipoprotein E deficiency. J Clin Invest 78:1206–1219
Lessner SM, Prado HL, Waller EK, Galis ZS (2002) Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol 160:2145–2155
Pendse AA, rbones-Mainar JM, Johnson LA, Altenburg MK, Maeda N (2009) Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res 50(Suppl):S178–S182
Reddick RL, Zhang SH, Maeda N, Atherosclerosis in mice lacking apo E (1994) Evaluation of lesional development and progression. Arterioscler Thromb 14:141–147
Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H (2009) Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297:H1535–H1543
Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 49:2379–2393
Korshunov VA, Berk BC (2003) Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol 23:2185–2191
Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H (2010) Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116:e66–e73
Singla DK, Lyons GE, Kamp TJ (2007) Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol 293:H1308–H1314
Zhou J, Lhotak S, Hilditch BA, Austin RC (2005) Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111:1814–1821
Cheng C, Tempel D, van HR, van der BA, Grosveld F, Daemen MJ, Krams R, de CR (2006) Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753
Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P (2006) Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol 290:H2320–H2328
Asakura T, Karino T (1990) Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res 66:1045–1066
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5:293–302
Moore JE Jr, Xu C, Glagov S, Zarins CK, Ku DN (1994) Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110:225–240
Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL (2003) Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 108:438–444
Wentzel JJ, Krams R, Schuurbiers JC, Oomen JA, Kloet J, van der Giessen WJ, Serruys PW, Slager CJ (2001) Relationship between neointimal thickness and shear stress after Wallstent implantation in human coronary arteries. Circulation 103:1740–1745
Wentzel JJ, Corti R, Fayad ZA, Wisdom P, Macaluso F, Winkelman MO, Fuster V, Badimon JJ (2005) Does shear stress modulate both plaque progression and regression in the thoracic aorta? Human study using serial magnetic resonance imaging. J Am Coll Cardiol 45:846–854
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145:341–355
Wolfs IM, Donners MM, de Winther MP (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost 106:763–771
Li YH, Hsieh CY, Wang DL, Chung HC, Liu SL, Chao TH, Shi GY, Wu HL (2007) Remodeling of carotid arteries is associated with increased expression of thrombomodulin in a mouse transverse aortic constriction model. Thromb Haemost 97:658–664
Parzy E, Miraux S, Franconi JM, Thiaudiere E (2009) In vivo quantification of blood velocity in mouse carotid and pulmonary arteries by ECG-triggered 3D time-resolved magnetic resonance angiography. NMR Biomed 22:532–537
Acknowledgments
The authors would like to thank Dr. Binbin Yan, Reetu Singla, and Latifa Abdelli for their assistance in cell counting, confocal immunostaining, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merino, H., Parthasarathy, S. & Singla, D.K. Partial ligation-induced carotid artery occlusion induces leukocyte recruitment and lipid accumulation—A shear stress model of atherosclerosis. Mol Cell Biochem 372, 267–273 (2013). https://doi.org/10.1007/s11010-012-1468-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1468-7