Abstract
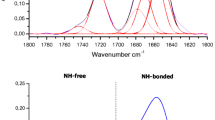
β-Amino acids with side chains at C2 and/or at C3 are of growing interest in drug design, as they may induce astonishing and unusual peptide conformations. Therefore it is of eminent importance to gather information on the consequences of β-amino acid incorporation on the three-dimensional structure of a peptide. This paper describes the synthesis and conformational analysis of cyclic penta- and hexapeptides comprising either (S)-Pro or (S)-β-Hpro. The conformational influence of the β-homoproline building block was analyzed by the combined application of CD, FT-IR and NMR. While the CD spectra of the proline containing peptides indicate the presence of inverse γ-turns and βII-turns, the CD spectra of the β-homoamino acid analogs are dominated by an unprecedented negative band near 205 nm associated with a pseudo-β-turn (Ψβ) or pseudo-γ-turn (Ψγ). These results were confirmed by FT-IR spectroscopy, which also indicates the formation of two internal hydrogen bonds in the cyclic peptides containing the β-homoproline. The conformations of the β-homoproline containing pentapeptides were additionally determined by NMR in combination with MD simulations in two different solvents. The conformation in trifluoroethanol (TFE) is characterized by a bifurcated hydrogen bond stabilizing a pseudo-γ-turn with β-homoproline in the central position, nested with a pseudo-β-turn with β-homoproline in the i+1 position. The combined CD/FT-IR studies clearly show that the replacement of proline by β-homoproline gives rise to a more flexible peptide backbone, and CD spectroscopy hints towards the presence of pseudo-β- or pseudo-γ-turns.






Similar content being viewed by others
References
Bax A., Davis D. G. (1985) J. Magn. Res. 65:355–360
Daura X., van Gunsteren W. F., Mark A. E. (1999) Proteins: Struct., Funct., Genet. 34:269–280
Fasman G. D. (1996). Circular Dichroism, the Conformational Analysis of Biomolecules Plenum Press New York
Fioroni M., Burger K., Mark A. E., Roccatano D. (2000) J. Phys. Chem. B 104:12347–12354
Gellman S. H. (1998) Acc. Chem. Res. 31:173–180
Goddard, T. D. and Kneller, D. G.: SPARKY 3. University of Califonia, San Francisco. http://www.cgl.ucsf.edu/home/sparky/
Lloyd-Williams P., Albericio F., Giralt E. (1997) Chemical Approaches to the Synthesis of Peptides and Proteins CRC Press Boca Raton and references cited there in
Macura S., Huang Y., Suter D., Ernst R. R. (1981) J. Magn. Res. 43:259–281
Madison V., Kopple K. D. (1980) J. Am. Chem. Soc. 102:4855–4863
Malešević M., Strijowski U., Bächle D., Sewald N. (2004) J. Biotechnol. 112:73–77
Martinek T. A., Tóth G. K., Vass E., Hollósi M., Fülöp F. (2002) Angew. Chem. 114:1794–1797 Angew. Chem. Int. Ed. 45, 1718–1721
Müller, A., Vogt, C. and Sewald, N.: 1998, Synthesis of Fmoc-β-Homoamino Acids by Ultrasound-Promoted Wolff Rearrangement. Synthesis, 837–841
Muñoz-Guerra, S., López-Carrasquero, F., Fernández-Santín, J. M. and Subirana, J. A.: 1996, Polymeric Materials Encyclopedia, Vol. 6, J. C. Salomone (ed.), CRC Press, Boca Raton, pp. 4694–4700
Pardi A., Billeter M., Wüthrich K. (1984) J. Mol. Biol. 180:741–751
Perczel, A. and Hollósi, M.: 1996, Circular Dichroism and the Conformational Analysis of Biomolecules. G. D. Fasman (ed.), Plenum Press, New York, pp. 285–367
Rance M., Sørensen O. W., Bodenhausen G., Wagner G., Ernst R. R., Wüthrich K. (1983) Biochem. Biophys. Res. Commun. 117:479–485
Rychaert J.-P., Ciccotti G., Berendsen H. J. (1977) J. Comput. Phys. 23:327–341
Schreiber J. V., Frackenpohl J., Moser F., Fleischmann T., Kohler H.-P. E., Seebach D. (2002) ChemBioChem 3:424–432
Schuler L. D., Daura X., van Gunsteren W. F. (2001) J. Comput. Chem. 22:1205–1218
Schumann F., Müller A., Koksch M., Müller G., Sewald N. (2000) J. Am. Chem. Soc. 122:12009–12010
Schwieters C. D., Kuszewski J. J., Tjandra N., Clore G. M. (2003) J. Magn. Res., 160:65–73
Seebach D., Beck A. K., Bierbaum D. J. (2004) Chem. Biodivers. 1:1111–1239
Shaka A. J., Lee C. J., Pines A. (1988) J. Magn. Res. 77:274–293
Strijowski U., Sewald N. (2004) Org. Biomol. Chem. 2:1105–1109
Surewicz W. K., Mantsch H. H. (1988) Biochim. Biophys. Acta 952:115–130
van Gunsteren W. F., Krüger P., Billeter S. R., Mark A. W., Eising A. A., Scott W. R. P., Hünenberger P. H., Tironi I. G. (1996) Biomolecular Simulation: The GROMOS96 Manual and User Guide vdf Hochschulverlag, ETH Zürich Switzerland
Vass E., Kurz M., Konat R. K., Hollósi M. (1998) Spectrochim. Acta Part A 54:773–786
Vass E., Hollósi M., Besson F., Buchet R. (2003) Chem. Rev. 103:1917–1954
Acknowledgments
This project was supported by Deutsche Forschungsgemeinschaft (Germany, SFB 549 Macromolecular Processing and Signalling in the Extracellular Matrix), Deutscher Akademischer Austauschdienst (PPP), OTKA (Hungary, projects number T037719 and T 047186), and the Fonds der Chemischen Industrie (Frankfurt, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malešević, M., Majer, Z., Vass, E. et al. Spectroscopic Detection of Pseudo-Turns in Homodetic Cyclic Penta- and Hexapeptides Comprising β-Homoproline. Int J Pept Res Ther 12, 165–177 (2006). https://doi.org/10.1007/s10989-006-9013-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-006-9013-8