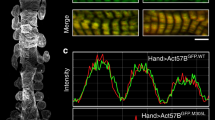
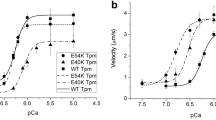
Abstract
Striated muscle contraction is regulated primarily through the action of tropomyosin and troponin that are bound to actin. Activation requires Ca2+ binding to troponin and/or binding of high affinity myosin complexes to actin. Mutations within components of the regulatory complex may lead to familial cardiomyopathies and myopathies. In several cases examined, either physiological or pathological changes in troponin alter the distribution among states of actin–tropomyosin–troponin that differ in their abilities to stimulate myosin ATPase activity. These observations open possibilities for managing disorders of the troponin complex. Furthermore, analyses of mutant forms of troponin give insights into the regulation of striated muscle contraction.


Similar content being viewed by others
Abbreviations
- S1:
-
Myosin subfragment 1
- NEM-S1:
-
S1 modified with N-ethylmaleimide
- Regulated actin:
-
The complex of actin–tropomyosin and troponin
References
Borrego-Diaz E, Chalovich JM (2010) Kinetics of regulated actin transitions measured by probes on tropomyosin. Biophys J 98:2601–2609
Brady PA (2012) Surviving hypertrophic cardiomyopathy: counting the cost of implantable cardioverter defibrillator therapy to prevent sudden death. Heart 98:97–98
Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ (2003) Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem 35:1285–1293
Chalovich JM (2002) Regulation of striated muscle contraction: a discussion. J Muscle Res Cell Motil 23:353–361
Chalovich JM, Eisenberg E (1982) Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem 257:2432–2437
Chalovich JM, Greene LE, Eisenberg E (1983) Crosslinked myosin subfragment 1: a stable analogue of the subfragment-1-ATP complex. Proc Natl Acad Sci USA 80:4909–4913
Chalovich JM, Yan B, Brenner B, Chen YD (2002) Modeling thin filament cooperativity––Response. Biophys J 82:1679–1681
Chen Y, Yan B, Chalovich JM, Brenner B (2001) Theoretical kinetic studies of models for binding myosin subfragment-1 to regulated actin: Hill model versus Geeves model. Biophys J 80:2338–2349
Eisenberg E, Kielley W (1970) Native tropomyosin: effect on the interaction of actin with heavy meromyosin and subfragment-1. Biochem Biophys Res Commun 40:50–56
Eisenberg E, Weihing RR (1970) Effect of skeletal muscle native tropomyosin on the interaction of amoeba actin with heavy meromyosin. Nature 228:1092–1093
El-Saleh SC, Potter JD (1985) Calcium-insensitive binding of heavy meromyosin to regulated actin at physiological ionic strength. J Biol Chem 260:14775–14779
Franklin AJ, Baxley T, Kobayashi T, Chalovich JM (2012) The C-terminus of troponin T is essential for maintaining the inactive state of regulated actin. Biophys J 102:2536–2544
Gafurov B, Chalovich JM (2007) Equilibrium distribution of skeletal actin-tropomyosin-troponin states, determined by pyrene-tropomyosin fluorescence. FEBS J 274:2287–2299
Gafurov B, Chen YD, Chalovich JM (2004a) Ca2+ and ionic strength dependencies of S1-ADP binding to actin-tropomyosin-troponin: regulatory implications. Biophys J 87:1825–1835
Gafurov B, Fredricksen S, Cai A, Brenner B, Chase PB, Chalovich JM (2004b) The Δ14 mutation of human cardiac troponin T enhances ATPase activity and alters the cooperative binding of S1-ADP to regulated actin. Biochemistry 43:15276–15285
Golitsina N, An YM, Greenfield NJ, Thierfelder L, Iizuka K, Seidman JG, Seidman CE, Lehrer SS, Hitchcock-DeGregori SE (1997) Effects of two familial hypertrophic cardiomyopathy-causing mutations on α-tropomyosin structure and function. Biochemistry 36:4637–4642
Greene LE (1986) Cooperative binding of myosin subfragment one to regulated actin as measured by fluorescence changes of troponin I modified with different fluorophores. J Biol Chem 261:1279–1285
Greene LE, Williams DL Jr, Eisenberg E (1987) Regulation of actomyosin ATPase activity by troponin-tropomyosin: effect of the binding of the myosin subfragment 1 (S-1)·ATP complex. Proc Natl Acad Sci USA 84:3102–3106
Haselgrove JC (1972) X-ray evidence for a conformational change in the actin containing filaments of vertebrate striated muscle. Cold Spring Harb Symp Quant Biol 37:341–352
Heeley DH, Belknap B, White HD (2002) Mechanism of regulation of phosphate dissociation from actomyosin-ADP-Pi by thin filament proteins. Proc Nat Acad Sci USA 99:16731–16736
Heeley DH, Belknap B, White HD (2006) Maximal activation of skeletal muscle thin filaments requires both rigor myosin S1 and calcium. J Biol Chem 281:668–676
Hill TL, Eisenberg E, Greene LE (1980) Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci USA 77:3186–3190
Hill TL, Eisenberg E, Chalovich JM (1981) Theoretical models for cooperative steady-state ATPase activity of myosin subfragment-1 on regulated actin. Biophys J 35:99–112
Huxley HE (1972) Structural changes in the actin and myosin containing filaments during contraction. Cold Spring Harb Symp Quant Biol 37:361–376
Ishii Y, Lehrer SS (1993) Kinetics of the “on-off” change in regulatory state of the muscle thin filament. Arch Biochem Biophys 305:193–196
Karibe A, Tobacman LS, Strand J, Butters C, Back N, Bachinski LL, Arai AE, Oritz A, Roberts R, Homsher E, Fananapazir L (2007) Hypertrophic cardiomyopathy caused by a novel alpha-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis pp 65–71
Kraft T, Chalovich JM, Yu LC, Brenner B (1995) Parallel inhibition of active force and relaxed fiber stiffness by caldesmon fragments at physiological ionic strength and temperature conditions: additional evidence that weak cross-bridge binding to actin is an essential intermediate for force generation. Biophys J 68:2404–2418
Kremneva E, Boussouf S, Nikolaeva O, Maytum R, Geeves MA, Levitsky DI (2004) Effects of two familial hypertrophic cardiomyopathy mutations in {alpha}-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin. Biophys J 87:3922–3933
Malhotra A, Scheuer J, Patel K, Lopez MC (1990) Troponin-tropomyosin abnormalities in hamster cardiomyopathy. J Clin Invest 86:286–292
Massin EK (1998) Treatment of advanced heart failure in a young man with familial cardiomyopathy. Tex Heart Inst J 25:294–297
Mathur MC, Kobayashi T, Chalovich JM (2008) Negative charges at protein kinase C sites of troponin I stabilize the inactive state of actin. Biophys J 94:542–549
Mathur MC, Kobayashi T, Chalovich JM (2009) Some cardiomyopathy causing troponin I mutations stabilize a functional intermediate actin state. Biophys J 96:2237–2244
Mathur MC, Chase PB, Chalovich JM (2011) Several cardiomyopathy causing mutations on tropomyosin either destabilize the active state of actomyosin or alter the binding properties of tropomyosin. Biochem Biophys Res Commun 406:74–78
McKillop DFA, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701
Meeusen RL, Cande ZW (1979) N-ethylmaleimide-modified heavy meromyosin. A probe for actomyosin interactions. J Cell Biol 82:57–65
Moir AJ, Solaro RJ, Perry SV (1980) The site of phosphorylation of troponin I in the perfused rabbit heart. Eff adrenaline Biochem J 185:505–513
Murray JM, Knox MK, Trueblood CE, Weber A (1982) Potentiated state of the tropomyosin actin filament and nucleotide-containing myosin subfragment 1. Biochemistry 21:906–915
Parry DAD, Squire JM (1973) Structural role of tropomyosin in muscle regulation: analysis of the X-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol 75:33–55
Pemrick S, Weber A (1976) Mechanism of inhibition of relaxation by N-ethylmaleimide treatment of myosin. Biochemistry 15:5193–5198
Pirani A, Xu C, Hatch V, Craig R, Tobacman LS, Lehman W (2005) Single particle analysis of relaxed and activated muscle thin filaments. J Mol Biol 761–772
Poole KJV, Lorenz M, Evans G, Rosenbaum G, Pirani A, Craig R, Tobacman LS, Lehman W, Holmes KC (2006) A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol 155:273–284
Potter JD, Sheng Z, Pan B-S, Zhao J (1995) A direct regulatory role for troponin T and a dual role for troponin C in the Ca2+ regulation of muscle contraction. J Biol Chem 270:2557–2562
Resetar AM, Stephens JM, Chalovich JM (2002) Troponin-tropomyosin: an allosteric switch or a steric blocker? Biophys J 83:1039–1049
Ribolow H, Bárány K, Steinschneider A, Bárány M (1977) Lack of phosphate incorporation into TN-I in live frog muscle. Arch Biochem Biophys 179:81–85
Rosenfeld SS, Taylor EW (1987) The mechanism of regulation of actomyosin subfragment 1 ATPase. J Biol Chem 262:9984–9993
Shitaka Y, Kimura C, Miki M (2005) The rates of switching movement of troponin T between three states of skeletal muscle thin filaments determined by fluorescence resonance energy transfer. J Biol Chem 280:2613–2619
Smith DA, Maytum R, Geeves MA (2003) Cooperative regulation of myosin-actin interactions by a continuous flexible chain I: actin-tropomyosin systems. Biophys J 84:3155–3167
Tardiff JC (2011) Thin filament mutations: developing an integrative approach to a complex disorder. Circ Res 108:765–782
Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg H-P, Seidman JG, Seidman CE (1994) α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77:701–712
Trybus KM, Taylor EW (1980) Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci USA 77:7209–7213
Varughese JF, Chalovich JM, Li Y (2010) Molecular dynamics studies on troponin (TnI-TnT-TnC) complexes: insight into the regulation of muscle contraction. J Biomol Struct Dyn 28:159–174
Varughese JF, Baxley T, Chalovich JM, Li Y (2011) A computational and experimental approach to investigate bepridil binding with cardiac troponin. J Phys Chem B 115:2392–2400
Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE (1995) Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332:1058–1064
Author information
Authors and Affiliations
Corresponding author
Additional information
I last spoke with Michael Barany in 2008 at the University of Illinois School of Medicine. He encouraged me to write a review on the topic of a seminar that I had just given in the Department of Physiology & Biophysics. This brief review honors that request.
Rights and permissions
About this article
Cite this article
Chalovich, J.M. Disease causing mutations of troponin alter regulated actin state distributions. J Muscle Res Cell Motil 33, 493–499 (2012). https://doi.org/10.1007/s10974-012-9305-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-012-9305-x