Abstract
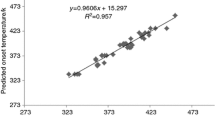
Organic peroxides are widely used unstable compounds that have caused many serious industrial incidents. Self-accelerating decomposition temperature (SADT) is one of the most important parameters to describe the thermal instability hazards of organic peroxides. However, it is very difficult to obtain experimental data of SADT due to high cost, the time involved and safety issues of laboratory tests. Quantitative structure–property relationship (QSPR) models have been proposed as an effective tool to predict thermal stability of organic peroxides. In this work, a dataset including 50 SADTs of organic peroxides was built and their molecular descriptors were calculated at B3LYP/6-31G(d) level using Gaussian 09. Two novel predictive models were successfully developed by multiple linear regression (MLR) and support vector machine (SVM). Both models were validated to have an excellent goodness of fit, internal robustness and external predictive ability. The MLR model was a linear equation with the average absolute error of training set and test set being 9.78 and 9.91, while the SVM model was a nonlinear model with the two values being 4.33 and 5.75, respectively. The SVM model has higher accuracy and is much more effective than the MLR model. This research provides general guidelines and methodology of establishing QSPR models to predict SADTs for other organic peroxides and unstable hazardous chemicals.




Similar content being viewed by others
References
Wang YW, Liao MS, Shu CM. Thermal hazards of a green antimicrobial peracetic acid combining DSC calorimeter with thermal analysis equations. J Therm Anal Calorim. 2015;119(3):2257–67.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J Hazard Mater. 2009;171(1–3):1145–9.
Lee MH, Chen JR, Das M, Hsieh TF, Shu CM. Thermokinetic parameter evaluation by DSC and TAM III along with accountability of mass loss by TG from the thermal decomposition analyses of benzoyl peroxide. J Therm Anal Calorim. 2015;122(3):1143–50.
Chen WT, Chen WC, You ML, Tsai YT, Shu CM. Evaluation of thermal decomposition phenomenon for 1,1-bis(tertbutylperoxy)-3,3,5-trimethylcyclohexane by DSC and VSP2. J Therm Anal Calorim. 2015;122(3):1125–33.
Lu Y, Ng D, Miao L, Mannan MS. Key observations of cumene hydroperoxide concentration on runaway reaction parameters. Thermochim Acta. 2010;501(1–2):65–71.
Liu SH, Hou HY, Chen JW, Weng SY, Lin YC, Shu CM. Effects of thermal runaway hazard for three organic peroxides conducted by acids and alkalines with DSC, VSP2, and TAM III. Thermochim Acta. 2013;566:226–32.
Pan Y, Zhang Y, Jiang J, Ding L. Prediction of the self-accelerating decomposition temperature of organic peroxides using the quantitative structure–property relationship (QSPR) approach. J Loss Prev Proc. 2014;31:41–9.
Foresman JB, Frisch AE. Exploring chemistry with electronic structure methods. 2nd ed. Gaussian Inc.: Pittsburgh, PA; 1996.
Wang Q, Zhang Y, Rogers WJ, Mannan MS. Molecular simulation studies on chemical reactivity of methylcyclopentadiene. J Hazard Mater. 2009;165(1–3):141–7.
Ochterski JW. Thermochemistry in Gaussian. Gaussian Inc.; 2000. http://www.lct.jussieu.fr/manuels/Gaussian03/g_whitepap/thermo/thermo.pdf. Accessed 2 June 2000.
Wang Q, Mannan MS. Prediction of thermochemical properties for gaseous ammonia oxide. J Chem Eng Data. 2010;55(11):5128–32.
Sun J, Li Y, Hasegawa K. A study of self-accelerating decomposition temperature (SADT) using reaction calorimetry. J Loss Prev Proc. 2001;14(5):331–6.
Bosch CM, Velo E, Recasens F. Safe storage temperature of peroxide initiators: prediction of self-accelerated decomposition temperature based on a runaway heuristics. Chem Eng Sci. 2001;56(4):1451–7.
Yu Y, Hasegawa K. Derivation of the self-accelerating decomposition temperature for self-reactive substances using isothermal calorimetry. J Hazard Mater. 1996;45(2–3):193–205.
Gao Y, Xue Y, Lü Z, Wang Z, Chen Q, Shi N, Sun F. Self-accelerating decomposition temperature and quantitative structure-property relationship of organic peroxides. Process Saf Environ Prot. 2015;94:322–8.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxides based on non-isothermal decomposition behavior. J Loss Prev Proc. 2003;16(5):411–6.
Prana V, Rotureau P, Fayet G, Andréc D, Hub S, Vicot P, Rao L, Adamoa C. Prediction of the thermal decomposition of organic peroxides by validated QSPR models. J Hazard Mater. 2014;276:216–24.
The PubChem Project. https://pubchem.ncbi.nlm.nih.gov/#opennewwindow.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian, Inc., Wallingford; 2010.
Reyes OJ, Patel SJ, Mannan MS. Quantitative structure property relationship studies for predicting dust explosibility characteristics (K st, P max) of organic chemical dusts. Ind Eng Chem Res. 2011;50(4):2373–9.
Wang Q, Wang J, Larranaga MD. Simple relationship for predicting onset temperatures of nitro compounds in thermal explosions. J Therm Anal Calorim. 2013;111(2):1033–7.
Wang Q, Ng D, Mannan MS. Study on the reaction mechanism and kinetics of the thermal decomposition of nitroethane. Ind Eng Chem Res. 2009;48(18):8745–51.
Lu Y, Ng D, Mannan MS. Prediction of the reactivity hazards for organic peroxides using the QSPR approach. Ind Eng Chem Res. 2011;50(3):1515–22.
Wang Q, Rogers WJ, Mannan MS. Thermal risk assessment and rankings for reaction hazards in process safety. J Therm Anal Calorim. 2009;98:225–33.
Vapnik V. The nature of statistical learning theory. 2nd ed. Berlin: Springer; 2013.
Vapnik VN, Vapnik V. Statistical learning theory. London: Wiley; 1998.
de Cerqueira Lima P, Golbraikh A, Oloff S, Xiao Y, Tropsha A. Combinatorial QSAR modeling of P-glycoprotein substrates. J Chem Inf Model. 2006;46(3):1245–54.
Fatemi MH, Gharaghani S. A novel QSAR model for prediction of apoptosis-inducing activity of 4-aryl-4-H-chromenes based on support vector machine. Bioorgan Med Chem. 2007;15(24):7746–54.
Fatemi MH, Gharaghani S, Mohammadkhani S, Rezaie Z. Prediction of selectivity coefficients of univalent anions for anion-selective electrode using support vector machine. Electrochim Acta. 2008;53(12):4276–82.
Niazi A, Jameh-Bozorghi S, Nori-Shargh D. Prediction of toxicity of nitrobenzenes using ab initio and least squares support vector machines. J Hazard Mater. 2008;151(2–3):603–9.
Pan Y, Jiang J, Wang R, Cao H, Cui Y. A novel QSPR model for prediction of lower flammability limits of organic compounds based on support vector machine. J Hazard Mater. 2009;168(2–3):962–9.
Alexander G, Alexander T. Beware of q2! J Mol Graph Model. 2002;20(4):269–76.
Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2011;2(3):27:1–27:27. http://www.csie.ntu.edu.tw/~cjlin/libsvm.
Acknowledgements
This research was financially supported by China Scholarship Council (CSC). The authors would like to thank the High Performance Computing Center at Oklahoma State University for software support and computing time. The authors also appreciate the support from the Dale F. Janes Endowed Professorship at Oklahoma State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Yi, H., Xu, K. et al. Prediction of the self-accelerating decomposition temperature of organic peroxides using QSPR models. J Therm Anal Calorim 128, 399–406 (2017). https://doi.org/10.1007/s10973-016-5922-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5922-8