Abstract
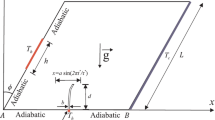
The flow and thermal behavior of the phase change material in a novel geometry, namely, quadrantal cavity, is investigated numerically. The influences of curved wall temperatures of 32, 37, and 42 °C, the initial sub-cooling temperatures of 5, 10, and 15 °C which are lower than ambient temperature, are examined previously. Inclination angles for the range from 0 to 360° on flow and thermal performances are carefully studied. It is found that the inclination angles affects dramatically not only the time of complete thermal energy storage but also convection currents. The best performance is obtained for an inclination angle of 225°.







Similar content being viewed by others
References
Albright G, Farid M, Al-Hallaj S. Development of a model for compensating the influence of temperature gradients within the sample on DSC-results on phase change materials. J Therm Anal Calorim. 2010;101:1155–60.
Agyenim F, Hewitt N, Eames P, Smyth M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew Sust Energ Rev. 2010;14:615–28.
Zivkovic B, Fujii I. An analysis of isothermal phase change of phase change material within rectangular and cylindrical containers. Sol Energy. 2001;70:51–61.
Saha SK, Dutta P. Heat transfer correlations for PCM-based heat sinks with plate fins. Appl Therm Eng. 2010;30:2485–91.
Talati F, Mosaffa AH, Rosen MA. Analytical approximation for solidification processes in PCM storage with internal fins: imposed heat flux. Heat Mass Transf. 2011;47:369–76.
Pal D, Joshi YK. Melting in a side heated tall enclosure by a uniformly dissipating heat source. Int J Heat Mass Transf. 2001;44:375–87.
Akgun M, Aydin O, Kaygusuz K. Thermal energy storage behavior of a paraffin during melting and solidification. Energy Source Part A. 2007;29:1315–26.
Akgun M, Aydin O, Kaygusuz K. Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Convers Manag. 2007;48:669–78.
Castell A, Belusko M, Bruno F, Cabeza LF. Maximisation of heat transfer in a coil in tank PCM cold storage system. Appl Energy. 2011;88:4120–27.
Shmueli H, Ziskind G, Letan R. Melting in a vertical cylindrical tube: Numerical investigation and comparison with experiments. Int J Heat Mass Transf. 2010;53:4082–91.
Assis E, Katsman L, Ziskind G, Letan R. Numerical and experimental study of melting in a spherical shell. Int J Heat Mass Transf. 2007;50:1790–804.
Tan FL, Hosseinizadeh SF, Khodadadi JM, Fan LW. Experimental and computational study of constrained melting of phase change materials (PCM) inside a spherical capsule. Int J Heat Mass Transf. 2009;52:3464–72.
Tyagi VV, Pandey AK, Kaushik SC, Tyagi SK. Thermal performance evaluation of a solar air heater with and without thermal energy storage. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1617-3.
Wang N, Zhang ZR, Zhu DS, Gao JW. The investigation of thermal conductivity and energy storage properties of graphite/paraffin composites. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1467-z.
Kao HT, Li M, Lv XW, Tan JM. Preparation and thermal properties of expanded graphite/paraffin/organic montmorillonite composite phase change material. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1520-y.
Mehling H, Cabeza LF. Heat and cold storage with PCM: an up to date introduction into basics and applications. Berlin: Springer-Verlag; 2008.
Voller VR, Cross M, Markatos NC. An enthalpy method for convection/diffusion phase change. Int J Numer Methods Eng. 1987;24:271–84.
Brent AD, Voller VR, Reid KJ. Enthalpy-porosity technique for modeling convection-diffusion phase change: application to the melting of a pure metal. Numer Heat Transf A Appl. 1988;13:297–318.
Zeng JL, Cao Z, Yang DW, Xu F, Sun LX, Zhang XF, Zhang L. Effects of MWNTs on phase change enthalpy and thermal conductivity of a solid-liquid organic PCM. J Therm Anal Calorim. 2009;95:507–12.
Gui XH, Lin B, Guo YX, Yuan XG. Two-dimensional transient thermal analysis of PCM canister of a heat pipe receiver under microgravity. Appl Therm Eng. 2011;31:735–41.
Guo CX, Zhang WJ. Numerical simulation and parametric study on new type of high temperature latent heat thermal energy storage system. Energy Convers Manag. 2008;49:919–27.
Fluent 6 User’s Guide, Fluent Inc, New Hampshire, USA, 2003.
Acknowledgements
This study is financially supported by the Key Science and Technology Park Support Program of Guangzhou Municipal of China (Project No. 2008Z1-1061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, WB., Zhu, DS. & Wang, N. Effect of the inclination angles on thermal energy storage in a quadrantal cavity. J Therm Anal Calorim 110, 1487–1492 (2012). https://doi.org/10.1007/s10973-011-2035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2035-2