Abstract
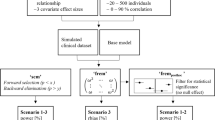
This study derives and assesses modified equations for Indirect Response Models (IDR) for normalizing data for baseline values (R 0) and evaluates different methods of utilizing baseline information. Pharmacodynamic response equations for the four basic IDR models were adjusted to reflect a ratio to, a change from (e.g., subtraction), or percent change relative to baseline. The original and modified IDR equations were fitted individually to simulated data sets and compared for recovery of true parameter values. Handling of baseline values was investigated using: estimation (E), fixing at the starting value (F1), and fixing at an average of starting and returning values of response profiles (F2). The performance of each method was evaluated using simulated data with variability under various scenarios of different doses, numbers of data points, type of IDR model, and degree of residual errors. The median error and inter-quartile range relative to true values were used as indicators of bias and precision for each method. Applying IDR models to normalized data required modifications in writing differential equations and initial conditions. Use of an observed/baseline ratio led to parameter estimates of k in = k out and inability to detect differences in k in values for groups with different R 0, whereas the modified equations recovered the true values. An increase in variability increased the %Bias and %Imprecision for each R 0 fitting method and was more pronounced for ‘F1’. The overall performance of ‘F2’ was as good as that of ‘E’ and better than ‘F1’. The %Bias in estimation of parameters SC50 (IC50) and k out followed the same trend, whereas use of ‘F1’ or ‘F2’ resulted in the least bias for S max (I max). The IDR equations need modifications to directly assess baseline-normalized data. In general, Method ‘E’ resulted in lesser bias and better precision compared to ‘F1’. With rich datasets including sufficient information on the return to baseline, Method ‘F2’ is reasonable. Method ‘E’ offers no significant advantage over ‘F1’ with datasets lacking information on the return to baseline phase. Handling baseline responses properly is an essential aspect of applying pharmacodynamic models.






Similar content being viewed by others
References
Schindel F (2000) Consideration of endogenous backgrounds in pharmacokinetic analyses: a simulation study. Eur J Clin Pharmacol 56:685–688
Colburn WA, Gibson DM (1988) Endogenous agonists and pharmacokinetic/pharmacodynamic modeling of baseline effects in current problems, potential solutions. In: Kroboth PD, Smith RB, Juhl RP (eds) Pharmacokinetics and pharmacodynamics, vol 2. Harvey Whitney Books, Cincinnati
Dansirikul C, Silber HE, Karlsson MO (2008) Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Pharmacodyn 35:269–283
Sun YN, Jusko WJ (1999) Role of baseline parameters in determining indirect pharmacodynamic responses. J Pharm Sci 88:987–990
D’Argenio DZ, Schumitzky A (1997) ADAPT II user’s guide: pharmacokinetic/pharmacodynamic system analysis software. Biomedical Simulations Resource, Los Angeles, CA
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Dayneka NL, Garg V, Jusko WJ (1993) Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 21:457–478
Yao Z, Krzyzanski W, Jusko WJ (2006) Assessment of basic indirect pharmacodynamic response models with physiological limits. J Pharmacokinet Pharmacodyn 33:167–193
Gozzi P, Pahlman I, Palmer L, Gronberg A, Persson S (1999) Pharmacokinetic-pharmacodynamic modeling of the immunomodulating agent susalimod and experimentally induced tumor necrosis factor-alpha levels in the mouse. J Pharmacol Exp Ther 291:199–203
Post TM, Freijer JI, DeJongh J, Danhof M (2005) Disease system analysis: basic disease progression models in degenerative disease. Pharm Res 22:1038–1049
Krzyzanski W, Chakraborty A, Jusko WJ (2000) Algorithm for application of Fourier analysis for biorhythmic baselines of pharmacodynamic indirect response models. Chronobiol Int 17:77–93
Chakraborty A, Krzyzanski W, Jusko WJ (1999) Mathematical modeling of circadian cortisol concentrations using indirect response models: comparison of several methods. J Pharmacokinet Biopharm 27:23–43
Ramanathan M (2002) A dispersion model for cellular signal transduction cascades. Pharm Res 19:1544–1548
Krzyzanski W, Dmochowski J, Matsushima N, Jusko WJ (2006) Assessment of dosing impact on intra-individual variability in estimation of parameters for basic indirect response models. J Pharmacokinet Pharmacodyn 33:635–655
Ahn JE, Karlsson MO, Dunne A, Ludden TM (2008) Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35:401–421
Acknowledgment
This work was funded by NIH grant GM57980.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: the impact of truncating negative values to a LLOQ in simulated datasets
Appendix 1: the impact of truncating negative values to a LLOQ in simulated datasets
Handling of observations below the LLOQ or negative values (in case of simulated data) in data analysis has been a matter of concern and investigation. A recent study which examined biexponential pharmacokinetic functions showed that truncation of normal distributions by simply ignoring or replacing them with the LLOQ led to model misspecification and biased parameter estimates [15]. The severity of such bias would be a function of various factors, including fraction of LLOQ adjustments, type and nature of model applied, and magnitude of random error. In analyses using simulated datasets, it would be best to prevent the occurrence of negative values in simulations either by re-parameterization (avoiding a negative values) or log-transformation. Otherwise, to avoid biased estimations, it was suggested to use originally simulated data even with negative values. However, in real life situations such as with measurement of most pharmacologic effects, negative values would likely not exist. In simulating IDR models with effects which fall below zero, common sense would seem to argue towards use of 0 or ½ LLOQ values since the fitted models would predict 0 as an actual lowest possible value (Models I and IV).
The data sets that were used in the main article included noise-added response values below zero. This seemed to be due to having an additive portion of residual error model, and the analyses were performed in the presence of such negative values. The portion of negative values in each data set varied depending upon type of IDR model and amount of residual error. The percentages of negative values in simulations were higher with residual error of 30%, at most 2–3% for IDR Models II, III, and IV. For the datasets simulated using IDR Model I, however, due to the nature of the model yielding a downward curve and maximum responses reaching near zero, up to 20% was noticed at RV = 30%. In order to assess the impact of truncation of negative values to a limit of detection, we performed additional analyses. The analyses were done for the same datasets, but we set those simulated negative values to 0.1 which was assumed to be the limit of detection.
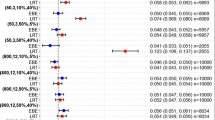
Table 6 summarizes values of bias of individual parameter estimates of Models I, II, III, and IV using the three estimation methods. For IDR Models II and III, the percentage of bias did not differ from those values obtained from the original datasets. There were noticeable differences in IDR Model I, especially in residual error of 30% as 20% of data was affected by the LLOQ. The data truncation in IDR Model I led to the overall bias being rather lower with 30% residual error than with 20% residual error, and Methods F1 and F2 having less bias than Method E, which was different from the typical trends that we observed from the other three models. However, even for the case of IDR Model I, when the baseline R0 is estimated, there was no difference. These changes occurred only in bias but not in precision (data not shown). For IDR Model IV, overall, the magnitudes of bias were downsized compared to those from data without the LLOQ, but the rank order of fitting methods was not changed and the trends remained the same, i.e., least bias in Method E followed by Method F2.
In summary, IDR Model I appeared to most affected by the data truncation in calculations of bias towards a higher residual error, which could lead to a different conclusion from the other cases. For the other IDR models, the magnitudes of bias and precision also changed in some scenarios. Nevertheless, the overall conclusions drawn from the current study regarding use of different baseline fitting methods remain the same in spite of this removal of negative values.
Rights and permissions
About this article
Cite this article
Woo, S., Pawaskar, D. & Jusko, W.J. Methods of utilizing baseline values for indirect response models. J Pharmacokinet Pharmacodyn 36, 381–405 (2009). https://doi.org/10.1007/s10928-009-9128-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-009-9128-6