Abstract
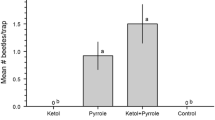
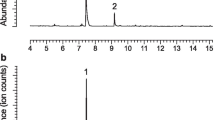
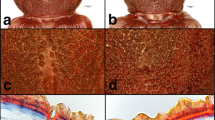
Males of several species of longhorned beetles in the subfamily Cerambycinae produce sex or aggregation pheromones consisting of 2,3-hexanediols and/or hydroxyhexanones. We tested the hypothesis that this diol/hydroxyketone pheromone motif is highly conserved within the subfamily, and the resulting prediction that multiple cerambycine species will be attracted to compounds of this type. We also tested the concept that live traps baited with generic blends of these compounds could be used as a source of live insects from which pheromones could be collected and identified. Traps placed in a mature oak woodland and baited with generic blends of racemic 2-hydroxyhexan-3-one and 3-hydroxyhexan-2-one captured adults of both sexes of three cerambycine species: Xylotrechus nauticus (Mannerheim), Phymatodes lecontei Linsley, and Phymatodes decussatus decussatus (LeConte). Odors collected from male X. nauticus contained a 9:1 ratio of two male-specific compounds, (R)- and (S)-3-hydroxyhexan-2-one. Field trials with synthetic compounds determined that traps baited with (R)-3-hydroxyhexan-2-one (94% ee), alone or in blends with other isomers, attracted similar numbers of X. nauticus of both sexes, whereas (S)-3-hydroxyhexan-2-one (94% ee) attracted significantly fewer beetles. Phymatodes lecontei and P. d. decussatus also were caught in traps baited with hydroxyhexanones, as well as a few specimens of two other cerambycine species, Neoclytus modestus modestus Fall (both sexes) and Brothylus gemmulatus LeConte (only females). Male N. m. modestus produced (R)-3-hydroxyhexan-2-one, which was not present in extracts from females. Neoclytus m. modestus of both sexes also responded to lures that included (R)-3-hydroxyhexan-2-one as one of the components. The only male-specific compound found in extracts from P. lecontei was (R)-2-methylbutan-1-ol, and adults of both sexes were attracted to racemic 2-methylbutan-1-ol in field bioassays. Surprisingly, P. lecontei of both sexes also were attracted to (R)- and (S)-3-hydroxyhexan-2-ones, although neither compound was detected in extracts from this species. Males of all five beetle species had gland pores on their prothoraces that were similar in structure to those that have been associated with volatile pheromone production in other cerambycine species. The attraction of multiple cerambycine species of two tribes to (R)-3-hydroxyhexan-2-one in this study, and in earlier studies with other cerambycine species, suggests that this compound is a widespread aggregation pheromone component in this large and diverse subfamily. Overall, the attraction of multiple species from different cerambycine tribes to this compound at a single field site supports the hypothesis that the hydroxyketone pheromone structural motif is highly conserved within this subfamily.





Similar content being viewed by others
References
Al Abassi, S., Birkett, M. A., Pettersson, J., Pickett, J. A., and Woodcock, C. M. 1998. Ladybird beetle odour identified and found to be responsible for attraction between adults. Cell. Mol. Life Sci. 54:876–879.
Bedard, W. D., Wood, D. L., Tilden, P. E., Lindahl, K. Q., Jr., Silverstein, R. M., and Rodin, J. O. 1980. Field responses of the western pine beetle and one of its predators to host- and beetle-produced compounds. J. Chem. Ecol. 6:625–641.
Cardé, R. and Haynes, K. F. 2004. Structure of the pheromone communication channel in moths, pp. 283–332, in R. T. Cardé and J. G. Millar (eds.). Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, United Kingdom.
Chemsak, J. 1963. Observations on the adult behavior of Xylotrechus nauticus. Pan-Pac. Entomol. 39:213–214.
Chénier, J. V. R. and Philogène, B. J. R. 1989. Field responses of certain forest Coleoptera to conifer monoterpenes and ethanol. J. Chem. Ecol. 15:1729–1745.
Dahlsten, D. L., Six, D. L., Rowney, D. L., Lawson, A. B., Erbilgin, N., and Raffa, K. F. 2004. Attraction of Ips pini (Coleoptera: Scolytinae) and its predators to natural attractants and synthetic semiochemicals in Northern California: Implications for population monitoring. Environ. Entomol. 33:1554–1561.
Dettner, K. and Reissenweber, F. 1991. The defensive secretion of Omaliinae and Proteininae (Coleoptera: Staphylinidae): its chemistry, biological and taxonomic significance. Biochem. Syst. Ecol. 19:291–303.
Eisner, T. and Meinwald, J. 2003. Alkaloid-derived pheromones and sexual selection in Lepidoptera, pp. 341–368, in G. J. Blomquist and R. G. Vogt (eds.). Insect Pheromone Biochemistry and Molecular Biology. Elsevier Academic Press, Amsterdam.
Fettköther, R., Dettner, K., Schröder, F., Meyer, H., Francke, W., and Noldt, U. 1995. The male pheromone of the old house borer Hylotrupes bajulus (L.) (Coleoptera: Cerambycidae): identification and female response. Experientia 51:270–277.
Haack, R. A. 2006. Exotic bark- and wood-boring Coleoptera in the United States: recent establishments and interceptions. Can. J. For. Res. 36:269–288.
Lacey, E. S., Ginzel, M. D., Millar, J. G., and Hanks, L. M. 2004. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Chem. Ecol. 30:1493–1507.
Lacey, E. S., Moreira, J. A., Millar, J. G., Ray, A. M., and Hanks, L. M. 2007a. Male-produced aggregation pheromone of the longhorned beetle Neoclytus mucronatus mucronatus. Entomol. Exp. Appl. 122:171–179.
Lacey, E. S., Ray, A. M., and Hanks, L. M. 2007b. Calling behavior of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Insect Behav. 20:117–128.
Leal, W. S. 1999. Scarab beetles, pp. 51–68, in J. Hardie and A. K. Minks (eds.). Pheromones of Non-lepidopteran Insects Associated with Agricultural Plants. CABI Publishing, Wallingford, UK.
Leal, W. S., Shi, X., Nakamuta, K., Ono, M., and Meinwald, J. 1995. Structure, stereochemistry, and thermal isomerization of the male sex pheromone of the longhorn beetle Anaglyptus subfasciatus. Proc. Natl. Acad. Sci. U. S. A. 92:1038–1042.
Lin, H. and Phelan, P. L. 1991. Identification of food volatiles attractive to Glischrochilus quadrisignatus and Glischrochilus fasciatus (Coleoptera: Nitidulidae). J. Chem. Ecol. 17:2469–2480.
Linsley, E. G. 1962. The Cerambycidae of North America, Part III: Taxonomy and classification of the subfamily Cerambycinae, Tribes Opsimini through Megaderini. Univ. Calif. Publ. Entomol. 21:1–163.
Linsley, E. G. 1964. The Cerambycidae of North America, Part V: Taxonomy and classification of the subfamily Cerambycinae, Tribes Callichromini through Ancylocerini. Univ. Calif. Publ. Entomol. 22:1–197.
McBrien, H. L., Millar, J. G., Gottlieb, L., Chen, X., and Rice, R. E. 2001. Male-produced sex attractant pheromone of the green stink bug, Acrosternum hilare. J. Chem. Ecol. 27:1821–1839.
Monné, M. A. and Hovore, F. T. 2005. Checklist of the Cerambycidae (Coleoptera) of the Western Hemisphere. BioQuip Publications, Rancho Dominguez, CA.
Moore, A. J., Reagan, M. L., and Haynes, K. F. 1995. Conditional signaling strategies—effects on ontogeny, social experience and social status on the pheromonal signal of male cockroaches. Anim. Behav. 50:191–202.
Polysciences. 2003. Poly/Bed® 812 Embedding Media/ DMP-30 Kit and Poly/Bed® 812 Embedding Media. Technical Data Sheet #233. Polysciences, Inc. Warrington, PA.
Pope, M. M., Gaston, L. K., and Baker, T. C. 1982. Composition, quantification, and periodicity of sex pheromone gland volatiles from individual Heliothis virescens females. J. Chem. Ecol. 8:1043–1055.
Ray, A. M., Lacey, E. S., and Hanks, L. M. 2006. Predicted taxonomic patterns in pheromone production by longhorned beetles. Naturwissenschaften 93:543–550.
Reddy, G. V. P., Fettköther, R., Noldt, U., and Detner, K. 2005. Capture of females Hylotrupes bajulus as influenced by trap type and pheromone blend. J. Chem. Ecol. 31:2169–2177.
Rochat, D., Meillour, P. N., Esteban-Duran, J. R., Malosse, C., Perthuis, B., Morin, J. P., and Déscoins, C. 2000. Identification of pheromone synergists in American palm weevil, Rhynchophorus palmarum, and attraction of related Dynamis borassi. J. Chem. Ecol. 26:155–187.
Sakakibara, Y., Yamane, A., Iwata, R., and Yamada, F. 1997. Evaluation of beetle capture in traps as compared with manual capture on flowers in a long-term investigation in a beech forest. J. For. Res. 2:233–236.
SAS Institute. 2001. SAS/STAT User’s Guide for Personal Computers, Release 8.01. SAS Institute, Cary, NC.
Schlyter, F. and Birgersson, G. A. 1999. Forest beetles, pp. 113–148, in J. Hardie and A. K. Minks (eds.). Pheromones of Non-Lepidopteran Insects Associated with Agricultural Plants. CABI Publishing, Wallingford, UK.
Schröder, F., Fettköther, R., Noldt, U., Dettner, K., König, W. A., and Franke, W. 1994. Synthesis of (3R)-3-hydroxy-2-hexanone, (2R,3R)-2,3-hexanediol and (2S,3R)-2,3-hexanediol, the male sex pheromone of Hylotrupes bajulus and Pyrrhidium sanguineum (Cerambycidae). Liebigs Ann. Chem. 1994:1211–1218.
Seybold, S. J., Teale, S. A., Wood, D. L., Zhang, A., Webster, F. X., Lindahl, K. Q., Jr., and Kubo, I. 1992. The role of lanierone in the chemical ecology of Ips pini (Coleoptera: Scolytidae) in California. J. Chem. Ecol. 18:2305–2329.
Sokal, R. R. and Rohlf, F. J. 1995. Biometry, 3rd ed. W. H. Freeman, New York, NY.
Solomon, J. D. 1995. Guide to Insect Borers in North American Broadleaf Trees and Shrubs. USDA For. Serv. Agr. Handbook 706.
Sweeney, J., Gutowski, J. M., Price, J., and De Groot, P. 2006. Effect of semiochemical release, killing agent, and trap design on detection of Tetropium fuscum (F.) and other longhorn beetles (Coleoptera: Cerambycidae). Environ. Entomol. 35:645–654.
Yanega, D. 1996. Field Guide to Northeastern Longhorned Beetles (Coleoptera: Cerambycidae). Manual 6, Illinois Natural History Survey, Urbana, IL.
Acknowledgments
We thank Frank Hovore (deceased), Douglas Yanega, and Ian Swift for assistance with identification of beetle species, Scott Robinson for help with SEM and histological sectioning work, Adam Martinsek for assistance with the statistical analysis, James Nardi for access to microscopy equipment, and Mariana Krugner and Ian Wright for technical assistance. We thank Carole Bell and Santa Rosa Plateau Reserve, Riverside Co., California, for access to field sites. The SEM and histology research was made possible through collaboration with Bugscope, The Imaging Technology Group, Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign (http://bugscope.beckman.uiuc.edu/). This project was supported by funds from the Exotic/Invasive Pests and Diseases Research Program, University of California, under USDA-CSREES Grant No. 2004-34439-14691, the Alphawood Foundation of Chicago, and Hatch Project #CA-R*ENT-5181H to JGM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanks, L.M., Millar, J.G., Moreira, J.A. et al. Using Generic Pheromone Lures to Expedite Identification of Aggregation Pheromones for the Cerambycid Beetles Xylotrechus nauticus, Phymatodes lecontei, and Neoclytus modestus modestus . J Chem Ecol 33, 889–907 (2007). https://doi.org/10.1007/s10886-007-9275-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9275-4