Abstract
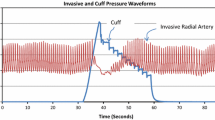
Commonly used arterial respiratory variation metrics are based on mathematical analysis of arterial waveforms in the time domain. Because the shape of the arterial waveform is dependent on the site at which it is measured, we hypothesized that analysis of the arterial waveform in the frequency domain might provide a relatively site-independent means of measuring arterial respiratory variation. Radial and femoral arterial blood pressures were measured in nineteen patients undergoing liver transplantation. Systolic pressure variation (SPV), pulse pressure variation (PPV), area under the curve variation (AUCV), and mean arterial pressure variation (MAPV) at radial and femoral sites were calculated off-line. Two metrics, “Spectral Peak Ratio” (SPeR) and “Spectral Power Ratio” (SPoR) based on ratios of the spectral peak and spectral area (power) at the respiratory and cardiac frequencies, were calculated at both radial and femoral sites. Variance among radial–femoral differences was compared and correlation coefficients describing the relationship between respiratory variation at the radial and femoral sites were developed. The variance in radial–femoral differences were significantly different (p < 0.001). The correlation between radial and femoral estimates of respiratory variation were 0.746, 0.658, 0.858, 0.882, 0.941, and 0.925 for SPV, PPV, AUCV, MAPV, SPeR, and SPoR, respectively. Assuming a PPV treatment threshold of 12 % (or equivalent), differences in treatment decisions based on radial or femoral estimates would arise in 12, 14, 5.4, 5.7, 4.8, and 5.5 % of minutes for SPV, PPV, AUCV, MAPV, spectral peak ratio, and spectral power ratio, respectively. As compared to frequency domain-based estimates of respiratory variation, SPV and PPV are relatively dependent on the anatomic site at which they are measured. Spectral peak and power ratios are relatively site-independent means of measuring respiratory variation, and may offer a useful alternative to time domain-based techniques.





Similar content being viewed by others
References
Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67:498–502.
Szold A, Pizov R, Segal E, Perel A. The effect of tidal volume and intravascular volume state on systolic pressure variation in ventilated dogs. Intensive Care Med. 1989;15:368–71.
Preisman S, DiSegni E, Vered Z, Perel A. Left ventricular preload and function during graded haemorrhage and retranfusion in pigs: analysis of arterial pressure waveform and correlation with echocardiography. Br J Anaesth. 2002;88:716–8.
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005;103:419–28 (quiz 49-5).
Auler JO, Jr, Galas F, Hajjar L, Santos L, Carvalho T, Michard F. Online monitoring of pulse pressure variation to guide fluid therapy after cardiac surgery. Anesth Analg 2008;106:1201–6 (table of contents).
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–21.
Deflandre E, Bonhomme V, Hans P. Delta down compared with delta pulse pressure as an indicator of volaemia during intracranial surgery. Br J Anaesth. 2008;100:245–50.
Hofer CK, Senn A, Weibel L, Zollinger A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care. 2008;12:R82.
Shin WJ, Choi JM, Kong YG, Song JG, Kim YK, Hwang GS. Spectral analysis of respiratory-related hemodynamic variables in simulated hypovolemia: a study in healthy volunteers with spontaneous breathing using a paced breathing activity. Korean J Anesthesiol. 2010;58:542–9.
O’Rourke MF. The arterial pulse in health and disease. Am Heart J. 1971;82:687–702.
Parker KH. A brief history of arterial wave mechanics. Med Biol Eng Comput. 2009;47:111–8.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–7.
Kleinman B. Understanding natural frequency and damping and how they relate to the measurement of blood pressure. J Clin Monit. 1989;5:137–47.
Efron B, Tibshirani R. An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993.
Rich GF, Lubanski RE Jr, McLoughlin TM. Differences between aortic and radial artery pressure associated with cardiopulmonary bypass. Anesthesiology. 1992;77:63–6.
Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY. Does radial artery pressure accurately reflect aortic pressure? Chest. 1992;102:1193–8.
Kim YK, Shin WJ, Song JG, Jun IG, Kim HY, Seong SH, Hwang GS. Comparison of stroke volume variations derived from radial and femoral arterial pressure waveforms during liver transplantation. Transpl Proc. 2009;41:4220–8.
Cannesson M, Awad AA, Shelley K. Oscillations in the plethysmographic waveform amplitude: phenomenon hides behind artifacts. Anesthesiology 2009;111:206–7 (author reply 7–8).
Magder S. Further cautions for the use of ventilatory-induced changes in arterial pressures to predict volume responsiveness. Crit Care. 2010;14:197.
Wyler von Ballmoos M, Takala J, Roeck M, Porta F, Tueller D, Ganter CC, Schroder R, Bracht H, Baenziger B, Jakob SM. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care 2010;14:R111.
Kronas N, Kubitz JC, Forkl S, Kemming GI, Goetz AE, Reuter DA. Functional hemodynamic parameters do not reflect volume responsiveness in the immediate phase after acute myocardial ischemia and reperfusion. J Cardiothorac Vasc Anesth. 2011;25(5):780–3
Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology. 2011;115:231–41.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiele, R.H., Colquhoun, D.A., Tucker-Schwartz, J.M. et al. Radial–femoral concordance in time and frequency domain-based estimates of systemic arterial respiratory variation. J Clin Monit Comput 26, 393–400 (2012). https://doi.org/10.1007/s10877-012-9390-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-012-9390-9