Abstract
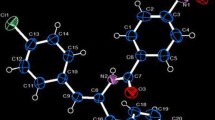
Starting from easily prepared (cyclobutylsulfonyl)benzene (1), a stereoselective synthesis of (±)-grandisol, accomplished in nine steps, with an overall yield of ca. 18 %, has been presented by Monteiro and Stefani (Eur J Org Chem 14:2659–2663, 2001). Most of the synthetic intermediates were secured in good to excellent yields as crystalline compounds requiring no or minimal purification, should being amenable to scale up. The structures and absolute stereochemistry of (2), (3), (4a), (5), (8) and (9) were established by IR and NMR (1H, 13C) spectroscopies and confirmed by X-ray diffraction analysis. Compound (2) crystallizes in orthorhombic Pbca, a = 16.0565(5), b = 9.5144(6), c = 23.9728(7) Å, the (3) crystallizes in monoclinic P21/c, a = 5.6390(5), b = 17.8630(16), c = 12.8678(12) Å and β = 111.928(7)°, the (4a) crystallizes in monoclinic P21/c, a = 5.7002(9) Å, b = 17.2752(14) Å, c = 14.9168(9) Å and β = 109.464(8)°. The other three cyclobutylsulfonyl derivatives crystallize in the same monoclinic space group P21/c with cell parameters (5) a = 8.072(4), b = 11.486(9), c = 14.565(8) Å and β = 101.373(4)°, (8) a = 11.3448(2), b = 7.9377(1), c = 18.5329(4) Å and β = 94.147(1)° and (9) a = 37.7571(9), b = 11.4434(3), c = 8.1824(2) Å and β = 90.748(1)°.
Graphical Abstract
X-ray crystal structures of six synthetic intermediates prepared from the stereoselective synthesis of (±)-grandisol.









Similar content being viewed by others
References
Tumlinson JH, Gueldner RC, Hardee DD, Thompson AC, Hedin PA, Minyard JP (1971) J Org Chem 36:2616–2621
Monteiro HJ, Stefani HA (2001) Eur J Org Chem 14:2659–2663
Bernard AM, Frongia A, Olliver J, Piras PP, Secci F, Spiga M (2007) Tetrahedron 63:4968–4974
Zarbin PHG, Villar JAFP, Corrêa AG (2007) J Braz Chem Soc 18:1100–1124
Francke W, Bartels J, Krohn S, Schultz S, Baader E, Tengö J, Schneider D (1989) Pure Appl Chem 61:539–542
Hooft RWW (1998) COLLECT: Nonius BV. Delft, The Netherlands
Otwinowski Z, Minor W (1997) In: Carter CW Jr, Sweet RM (eds) Processing of X-ray diffraction data collected in oscillation mode, methods in enzymology, macromolecular crystallography, part A, vol 276. Academic Press, New York, pp 307–326
Blessing RH (1995) Acta Cryst A51:33–38
Sheldrick GM (2008) Acta Cryst A64:112–122
Farrugia LJ (1997) J Appl Cryst 30:565
Farrugia LJ (1999) J Appl Cryst 32:837–838
Wilson ACJ (1992) Editor international tables of crystallography, vol C, mathematical, physical and chemical tables. Kluwer Academic Publishers, Dordrecht, p 693
Zukerman SJ, Monteiro HJ (1998) Acta Cryst C54:1673–1675
Zukerman SJ, Monteiro HJ (1998) Acta Cryst C54:96–97
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Pauling L (1960) The nature of the chemical bonding, 3rd edn. Cornell University, Ithaca, New York
Silverstein RM, Webster FX (2000) Identificação espectrométrica de compostos orgânicos, 6ª edição. Livros Técnicos e Científicos, Rio de Janeiro, p 99
Acknowledgments
This work was sponsored by grants from CNPq and FINEP (CT-INFRA 0970/01). GFS also gratefully acknowledges the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Edital Universal-2007, Processo 307412/2008-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa, G.F., Monteiro, H.J., Resck, I.S. et al. X-ray Crystal Structures of Intermediates of the Stereoselective (±)-Grandisol Synthesis Based on the Remote Alkylation Protocol. J Chem Crystallogr 43, 240–249 (2013). https://doi.org/10.1007/s10870-013-0411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0411-4