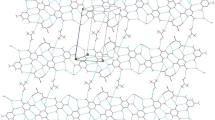
The aromatic derivative 2,8,14,20-tetranaphthylpyrogallol[4]arene was synthesized by the acid catalyzed condensation of 2-naphthaldehyde and pyrogallol in refluxing aqueous ethanol. Single crystal X-ray analysis revealed that the molecule crystallizes in a triclinic space group P1(bar) No. 2, with a = 11.3396(7) Å, b = 15.9942(10) Å, c = 26.3653(17) Å, α = 94.309(2)°, β = 91.765(2)°, γ = 93.892(2)°, D calc = 1.298 g/m3 for Z = 1. Within the unit cell, six methanol molecules of crystallization plus one molecule of pyrazine were found to accompany the pyrogallol macrocycle. In the solid state, the macrocycle is found to adopt the chair conformation.


Similar content being viewed by others
References
(a) Höberg, A.G. J. Am. Chem. Soc. 1980, 102, 6046. (b) Höberg, A.G. J. Org. Chem. 1980, 45, 4498.
For reviews see: (a) Container Molecules and Their Guests. Cram, D.J.; Cram, J.M.; Stoddart, F., Eds.; The Royal Society of Chemistry: London, 1994. (b) Timmerman, P.; Verboom, W.; Reinhoudt, D. Tetrahedron 1996, 52, 2663. (c) Rudkevich, D.; Rebek, J. Eur. J. Org. Chem. 1999, 1991. (d) Asfari, Z.; Böhmer, V.; Harrowfield, J.; Vicens, J.; Saadioui, M., Eds. Calixarenes 2001; Kluwer Academic: Dordrecht, The Netherlands, 2001.
(a) Cram, D.J.; Karbach, S.; Kim, H.E.; Knobler, C.B; Maverick, E.F.; Ericson, J.L.; Helgeson, R.C. J. Am. Chem. Soc. 1988, 110, 2229. (b) Cram, D.J.; Stewart, K.D.; Goldberg, I.; Trueblood, K.N. J. Am. Chem. Soc. 1985, 107, 2574.
Amrhein, P.; Shivanyuk, A.; Johnson, D.W.; Rebek, J. J. Am. Chem. Soc. 2002, 124, 10349.
(a) Rafari Far, A.; Rudkevich, D.; Haino, T.; Rebek, J. Org. Lett. 2000, 22, 3465. (b) Redshaw, C. Coord. Chem. Rev. 2003, 244, 45–70.
(a) Pirondini, L.; Bonifazi, D.; Menozzi, E.; Wegelius, E.; Rissanen, K.; Massera, C.; Dalcanale, E. Eur. J. Org. Chem. 2001, 12, 2311. (b) Sakhaii, P.; Neda, I.; Freytag, M.; Thonnessen, H.; Jones, P. G.; Schmutzler, R. Z. Anorg. Allg. Chem. 2000, 626, 1246. (c) Botta, B.; Delle, M.; Giuliano, R.; Paola, Z.G.; Seri, C.; Gacs-Baitz, E.; Csokasi, P.; Misiti, D. Eur. J. Org. Chem. 2000, 5, 841. (d) Lutzen, A.; Hass, O.; Bruhn, T. Tetrahedron Lett. 2002, 43, 1807.
(a) Nikolelis, D.P.; Petropoulou, S.E.; Theoharis, G. Electroanal. Chem. 2003, 15, 1616. (b) Collyer, S.D.; Davis, F.; Lucke, A.S.,; Charles, J.M.; Higson, S.P.J. J. Electroanal. Chem. 2003, 549, 119. (c) Kunsagi-Mate, S.; Nagy, L.; Nagy, G.; Bitter, I.; Kollar, L. J. Phys. Chem. B 2003, 107, 4727.
(a) Park, S.; Shin, D.; Sakamoto, S.; Yamaguchi, K.; Chung, Y.; Lah, M.; Hong, J. J. Chem. Soc. Chem. Commun. 2003, 8, 998. (b) Avram, L.; Cohen, Y. Org. Lett. 2002, 4, 4365. (c) Avram, L.; Cohen, Y. Org. Lett. 2003, 5, 4365, 1099. (d) Mustafina, A.; Skripacheva, V.; Kazakova, E.; Markarova, N.; Kataev, V.; Ermolaeva, L.; Habicher, W.D. J. Inclusion Phenom. Macro. Chem. 2002, 42, 77. (e) Mustafina, A.R.; Fedorenko, S.V.; Makarova, A.; Kazakova, E.; Bazhanova, Z.; Kataev, V.; Konovalov, A. J. Inclusion Phenom. Macro. Chem. 2001, 40, 73.
(a) Bibal, B.; Tinant, B.; Declercq, J.; Dutasta, J. Supramol. Chem. 2003, 15, 25. (b) Talanova, G. Ind. Eng. Chem. Res. 2000, 39, 3550. (c) Boerrigter, H.; Verboom, W.; Reinhoudt, D. J. Org. Chem. 1997, 62, 7148.
For C60/C70 complexes in solution see: (a) Haino, T.; Yanase, M.; Fukazawa,Y. Angew. Chem. Int. Ed. Engl. 1997, 36, 259. (b) Raston, C.; Atwood, J.; Nichols, P.; Sudria, I. Chem. Commun. 1996 2615. (c) Araki, K.; Akao, K.; Ikeda, A.; Suzuki, T.; Shinkai, S.; Tetrahedron Lett. 1996, 37, 73. (d) Ikeda, A.; Yoshimura, M.; Shinkai, S. Tetrahedron Lett. 1997, 38, 2107. (e) Ikeda, A.; Suzuki, Y.; Yoshimura, M.; Shinkai, S. Tetrahedron 1998, 54, 2497. (f) Atwood, J.; Barbour, L.; Raston, C.; Sudria, I. Angew. Chem. Int. Ed. Engl. 1998, 37, 981.
(a) Dalcanale, E.; Soncini, P.; Bacchilega, G.; Ugozzoli, F. J. Chem. Soc. Chem. Commun. 1989, 500. (b) Soncini, P.; Bonsignore, S.; Dalcanale, E.; Ugozzoli, F. J. Org. Chem. 1992, 57, 4608. (c) Dalcanale, E.; Costantini, G.; Soncini, P. J. Inclusion Phenom. Mol. Recognit. Chem. 1992, 13, 87.
For gas-phase complexation studies see: (a) Vincetti, M.; Dalcanale, E.; Soncini, P.; Guglielmetti, G. J. Am. Chem. Soc. 1990, 112, 445. (b) Vincetti, M.; Minero, C.; Pelizzetti, E.; Secchi, A.; Dalcanale, E. Pure Appl. Chem. 1995, 67, 1075. (c) Dickert, F.; Baumler, U.; Stathopulos, H. Anal. Chem. 1997, 69, 1000.
(a) Gibb, B.; Mezo, A.; Causton, A.; Fraser, J.; Tsai, F.; Sherman, J. Tetrahedron 1995, 51, 8719. (b) Fujimoto, T.; Shimizu, C.; Hayashida, O.; Aoyama, Y. J. Am. Chem. Soc. 1997, 119, 6676. (c) Fujimoto, T.; Shimizu, C.; Hayashida, O.; Aoyama, Y. J. Am. Chem. Soc. 1998, 120, 601. (d) Hayashida, O.T.; Shimizu, C.; Fujimoto, T.; Aoyama, Y. Chem. Lett. 1998, 13. (e) Aoyama, Y.; Matsuda, Y.; Chuleeraruk, J.; Nishiyama, K.; Fujimoto, K.; Fujimoto, T.; Shimizu, C.; Hayashida, O. Pure Appl. Chem. 1998, 70, 2379.
See reference 2d chapter 8.
(a) Li, X; Upton, T.; Gibb, C.; Gibb, B. J. Am. Chem. Soc. 2003, 125, 650. (b) Mustafina, A.; Skripacheva, V.; Kazakova, E.; Markarova, N.; Kataev, V.E.; Ermolaeva, L.V.; Habicher, W. J. Inclusion Phenom. Macro. Chem. 2002, 42, 77. (c) Wright, A.; Matthews, S.; Fischer, W.; Beer, P. Chem. Eur. J. 2001, 7, 3474.
(a) Ruderisch, A.; Pfeiffer, J.; Schurig, V. J. Chromatogr. A 2003, 994, 127. (b) Sokoliess, T.; Menyes, U.; Roth, U.; Jira, T. J. Chromatogr. A 2002, 948, 309.
(a) Ma, B.; Coppens, P. J. Chem. Soc. Chem. Commun. 2003, 4, 505. (b) Al'tshuler, G.N.; Fedyaeva, O.N.; Ostapova, E.V. Russ. Chem. Bull. 2000, 49, 1468. (c) Yamakawa, Y.; Ueda, M.; Nagahata, R.; Takeuchi, K.; Asai, M. J. Chem. Soc., Perkin Trans. 1 1998, 24, 4135.
SAINT. Version 6.02. Bruker AXS: Madison, WI, 1999.
Sheldrick, G.M., SHELXL-97; University of Gottingen: Germany, 1993.
Numbering sequence according to Gerkensmeier, T.; Iwanek, W.; Agena, C.; Frohlich, R.; Kotila, S.; Nather, C.; Mattay, J. Eur. J. Org. Chem. 199, 2257.
Halgren, T.A.; Nachbar, R.B. Merck Molecular Force Field. IV. Conformational Energies and Geometries for MMFF94. J. Comput. Chem. 1996, 17, 587–615.
Acknowledgments
The authors wish to thank Dr. Debra Bautista from Eastern Kentucky University for her help with the MOE calculations. Financial support for this work was obtained through the University Research Committee at Eastern Kentucky University (Grant No. 401062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary materials
CCDC 235410 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033.
Rights and permissions
About this article
Cite this article
Zambrano, C.H., Kass, J.P., Dueno, E.E. et al. Crystal structure of 2,8,14,20-tetranaphthylpyrogallol[4]arene. J Chem Crystallogr 36, 67–70 (2006). https://doi.org/10.1007/s10870-005-9028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-9028-6