Abstract
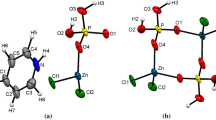
A new zinc(II) pyrophosphate, Zn4(P2O7)2 < eqid1 > ⋅10H2O, has been synthesized and characterized by single-crystal X-ray diffraction [orthorhombic space group Pnma, with unit cell parameters of a = 9.1508(2) A, b = 25.5271(5) Å, c = 8.3574(2) Å, Z = 4]. All the pyrophosphate anions show nonlinear P–O–P bonds with an average angle of 126.5∘. The framework of this new pyrophosphate is made up of packed layers of ZnO6 octahedra connected by double-tetrahedra P2O7 groups and a layer of Zn(H2O)6 units. The [P2O7]4− anions adopt a bent, near-staggered conformation. The absence of coincidences for the majority of the IR and Raman bands is in accord with the centrosymmetric structure of the material. The vibrational spectra have been interpreted in part on the basis of factor group effects. The structural changes occurring during heating have been investigated by TG-DSC, powder X-ray diffraction, and IR and Raman spectroscopy. When Zn4(P2O7)2 10H2O is gradually heated, it decomposes and γ-Zn2P2O7 is formed at 481∘C. On further heating, γ-Zn2P2O7 is transformed into β-Zn2P2O7 at 750∘C. The conversion between the γ and β-Zn2P2O7 forms is irreversible and, on cooling β-Zn2P2O7 to room temperature, it reverts back to α-Zn2P2O7. The crystal structure of the new zinc(II) pyrophosphate material is compared with the known structures of the related anhydrous products α-, β-, and γ-Zn2P2O7.
Similar content being viewed by others
References
Corbridge, D.E.C. The Structural Chemistry of Phosphorous; Elsevier: Amsterdam, 1974.
(a) Webb, N.C. Acta Crystallogr. (1966), 21, 942; (b) Calvo, C.; Au, P.K.L. Can. J. Chem. (1969), 47, 3409; (c) Krishnamachari, N.; Calvo, C. Acta Crystallogr. (1972), B28, 2883; (d) El Belghitti, A.E.; Boukhari, A.; Holt, E.M. Acta Crystallogr. (1994), C50, 482; (e) Kobashi, D.; Kohara, S.; Yamakawa, J.; Kawahara, A. Acta Crystallogr. (1997), C53, 1523; (f) El Bali, B.; Bolte, M. Acta Crystallogr. (2002), E58, 32; (g) Robertson, B.E.; Calvo, C. Acta Crystallogr. (1967), 22, 665; (h) Robertson, B.E.; Calvo, C. J. Solid State Chem. (1970), 1, 120; (i) Hoggins, J.T.; Swinnea, J.S.; Steinfink, H. J. Solid State Chem. (1983), 47, 278; (j) Weil, M.; Glaum, R. Acta Crystallogr. (1997), C53, 1000; (k) Calvo, C. Acta Crystallogr. (1967), 23, 289; (l) Tondon, V.K.; Calvo, C. Indian J. Pure Appl. Phys. (1981), 19, 756; (m) Stefanidis, T.; Nord, A.G. Acta Crystallogr. (1984), C40, 1995; (n) Lukaszewicz, K. Bull. Acad. Pol. Sci. Ser. Sci. Chem. (1967), 15, 47; (o) Pietraszko, A.; Lukaszewicz, K. Bull. Acad. Pol. Sci. Ser. Sci. Chem. (1968), 16, 183; (p) Mullica, D.F.; Perkins, H.O.; Grossie, D.A.; Boatnier, L.A.; Sales, B.C. J. Solid State Chem. (1986), 62, 371; (q) Hagman, L.O.; Jansson, I.; Magneli, C. Acta Chem. Scand. (1968), 22, 1419; (r) El Belghitti, A.E.; Boukhari, A.; Holt, E.M. Acta Crystallogr. (1994), C50, 482.
Capitelli, F.; Harcharras, M.; Assaaoudi, H.; Ennaciri, A.; Moliterni, A.G.G.; Bertolasi, V. Z. Kristallogr. (2003), 218, 345.
(a) Effenberger, H.; Pertlik, F. Monat. Für Chem. (1993), 124, 381; (b) Kongshaug, K.O.; Fjellvag, H.; Lillerud, K.P. Solid State Sci. (2000), 2, 205; (c) Schneider, S.; Collin, R.L. Inorg. Chem. (1973), 12, 2136
Mandel, N.S. Acta Crystallogr. (1975), B31, 1730.
Balic-Zunic, T.; Christoffersen, M.R.; Christoferssen, J. Acta Crystallogr. (2000), B56, 953.
Giesber, H.G. III; Korzenski, M.B.; Pennington, W.T.; Kolis, J.W. Acta Crystallogr. (2000), C56, 399.
Oka, J.; Kawahara, A. Acta Crystallogr. (1982), B38, 3.
Souhassou, M.; Lecomte, C.; Blessing, R.H. Acta Crystallogr. (1992), B48, 370.
Dojcilovic, J.; Novakovic, L.; Napijalo, M.M.; Napijalo, M.L. Mater. Chem. Phys. (1994), 39, 76.
Durif, A. Crystal Chemistry of Condensed Phosphates; Plenum: New York, 1995.
Calvo, C. Can. J. Chem. (1965), 43, 1147.
Bataille, T.; Bénard-Rocherullé, P.; Louër, D. J. Solid State Chem. (1998), 140, 62.
Nirsha, B.M.; Khomutova, T.V.; Fakeev, A.A.; Agre, V.M.; Zhadanov, B.V.; Allakhverdov, G.R.; Kozlova, N.P.; Olikova, V.A. Russ. J. Inorg. Chem. (Engl. Transl.) (1980), 25, 213.
Nirsha, B.M.; Khomutova, T.V.; Fakeev, A.A.; Agre, V.M.; Zhadanov, B.V.; Allakhverdov, G.R.; Kozlova, N.P.; Olikova, V.A. Zhurnal Neorganicheskoi Khimii (1980), 25(2), 391–5.
Quimby, M. Anal. Chem. (1957), 29, 248.
Corbridge, D.E.C. Acta Crystrallogr. (1957), 10, 85.
deWolff, Technisch Physische Dienst, Delft, Netherlands, ICDD Grant-in-Aid.
(a) Lazarev, A.N., Tenisheva, T.F. Izv. Akad. Nauk SSSR Ser. Khim. (1964), 3, 343; (b) Lazarev, A.N., Tenisheva, T.F. Izv. Akad. Nauk SSSR, Neorg. Mater. (1964), 5(1), 82; (c) Cornilsen, B.C., Condrate, R.A., Sr. J. Solid State Chem. (1978), 23, 375; (d) Cornilsen, B.C., Condrate, R.A., Sr. J. Phys. Chem. Solids (1977), 38, 1327; (e) Cornilsen, B.C.; Condrate, R.A., Sr. J. Inorg. Chem. (1979), 41, 602; (f) Steger, E.; Kassner, B. Spectrochim. Acta (1968), 24, 447.
Lavrov, A.V.; Bykanova, T.A. Neorg. Mater. (1979), 15(9), 1653; (b) Nirsha, N.B.; Khomutova, T.V.; Fakeev, A.A.; Agre, V.M.; Zhadanov, B.V.; Allakhverdov, G.R.; Kozlova, N.P.; Olikova, V.A. Russ. J. Inorg. Chem. (1980), 25(2), 213; (c) Kopilevich, V.A.; Shchegrov, L.N.; Panchuk, T.K. Russ. J. Inorg. Chem. (1993), 38(5), 734.
Harcharras, M., Ennaciri, A., Khay, N., Brouzi, K. J. Phosphorus, Silcon Sulfur (2003), 178(6), 1247.
Cornilsen, B.C. J. Mol. Struct. (1984), 117, 1.
Harcharras, M.; Ennaciri, A.; Capitelli, F.; Mattei, G. Vib. Spectrosc. (2003), 33, 189.
(a) Sheldrick, G.M. SHELXS97, Program for Crystal Structure Solution; University of Göttingen: Germany, 1997a; (b) Sheldrick, G.M. SHELXL97, Program for Crystal Structure Refinement; University of Göttingen: Germany, 1997b
Bruker. SHELXTL. Release 5.10, The Complete Software Package for Single Crystal Structure Determination; Bruker AXS: Madison, WI, 1997.
Burnett, M.N.; Johnson, C.K. ORTEP-III; Oak Ridge National Laboratory Report ORNL-6895, 1996.
Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. (1997), 30, 565.
Smith, T.J. Molview. J. Mol. Graphics (1995), 13, 122.
Ruszala, F.A.; Anderson, J.B.; Kostiner, E. Inorg. Chem. (1977), 16, 2417.
Mandel, N.S. Acta Crystallogr., Sect. B (1975), 31, 1730.
Kobashi, D.; Kohara, S.; Yamakawa, J.; Kawahara, A. Acta Crystallogr. (1997), C53, 1523.
Stranford, G.T., Condrate, R.A., Sr.; Cornilsen, B.C. J. Mol. Struct. (1981), 73, 231.
(a) Harcharras, M.; Ennaciri, A.; Assaaoudi, H.; Mattei, G.; D’Orazio, V.; Moliterni, A.G.G. J. Solid State Chem. (2003), 172, 160; (b) Harcharras, M.; Ennaciri, A.; Capitelli, F.; Mattei, G. Vib. Spectrosc. (2003), 33, 189.
Serrano, J.; Widulle, F.; Romero, A.H.; Rubio, A.; Lauck, R.; Cardona, M. Phys. Status Solidi B: Basic Res. (2003), 235(2), 260.
Kishioka, A.; Itatani, K.; Kinoshita, M. Yogyo-Kyokai-Shi (1985), 93(10), 606.
Katnack, F.L.; Hummel, F.A. J. Electrochem. Soc. (1958), 105(3), 125.
Petrova, M.A.; Shitova, V.I.; Mikirticheva, G.A.; Popova, V.F.; Malshikov, A.E. J. Solid State Chem. (1995), 119, 219.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assaaoudi, H., Butler, I.S., Kozinski, J. et al. Crystal structure, vibrational spectra and thermal decomposition of a new tetrazinc(II) dipyrophosphate decahydrate, Zn4(P2O7)2 < eqid1 > ⋅10H2O. J Chem Crystallogr 35, 49–59 (2005). https://doi.org/10.1007/s10870-005-1154-7
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-1154-7