Abstract
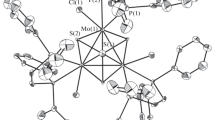
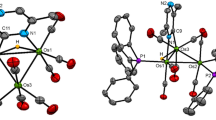
The heterometallic tetrahedrane clusters PhCCo2Ni(CO)6Cp (1) and PhCCo2Mo(CO)8Cp (3) react with the unsaturated diphosphine ligand (Z)-Ph2PCH=CHPPh2 at elevated temperature to afford the corresponding phosphine-substituted clusters PhCCo2Ni(CO)4[(Z)-Ph2PCH=CHPPh2]Cp (2) and PhCCo2Mo(CO)6[(Z)-Ph2PCH=CHPPh2]Cp (4). Clusters 2 and 4 have been isolated by column chromatography and characterized in solution by IR and NMR (1H and 31P) spectroscopies, and the solid-state structures of these clusters have been determined by X-ray crystallography. PhCCo2Ni(CO)4[(Z)-Ph2PCH=CHPPh2]Cp crystallizes in the triclinic space group P-l, a = 10.8142(7), b = 10.8994(6), c = 17.661(2) Å, α = 82.870(6), β = 86.149(7), γ = 64.493(5)∘, V = 1864.0(2) Å3, Z = 2, Dcacl = 1.495 g/cm3; R = 0.0409, Rw = 0.0424 for 3147 observed reflections with I > 3σ(I). PhCCo2Mo(CO)6[(Z)-Ph2PCH=CHPPh2]Cp, as the hexane solvent, crystallizes in the monoclinic space group P21/c, a = 12.706(4), b = 17.367(3), c = 21.639(7) Å, β = 106.48(3)∘, V = 4579(1) Å3, Z = 4, Dcacl = 1.478 g/cm3; R = 0.0605, Rw = 0.0724 for 1330 observed reflections with I > 3σ(I). The solid-state structures of 2 and 4 confirm a bridging coordination mode for the (Z)-Ph2PCH=CHPPh2 ligand, with adjacent cobalt centers being ligated by the ancillary phosphine ligand. The structural highlights of these new mixed-metal clusters are discussed relative to the homometallic tetrahedrane cluster PhCCo3(CO)7[(Z)-Ph2PCH=CHPPh2].
Similar content being viewed by others
References
Downard, A. J.; Robinson, B. H.; Simpson, J. Organometallics 1986, 5, 1122. Collin, J.; Jossart, C.;
Balavoine, G. Organometallics 1986, 5, 203.
Aime, S.; Botta, M.; Gobetto, R.; Osella, D. J. Organomet. Chem. 1987, 320, 229.
Yang, K.; Bott, S. G.; Richmond, M. G. J. Organomet. Chem. 1993, 454, 273.
Beurich, H.; Vahrenkamp, H. Chem. Ber. 1981, 114, 2542.
Don, M.-J.; Richmond, M. G.; Watson, W. H.; Krawiec, M.; Kashyap, R. P. J. Organomet. Chem. 1991, 418, 231.
Choi, N.; Conole, G.; King, J. D.; Mays, M. J.; McPartlin, M.; Stone, C. L. J. Chem. Soc. Dalton Trans. 2000, 395.
Bott, S. G.; Wang, J. C.; Richmond, M. G. J. Chem. Crystallogr. 1998, 28, 401.
Watson, W. H.; Nagl, A.; Hwang, S.; Richmond, M. G. J. Organomet. Chem. 1993, 445, 163.
Yang, K.; Smith, J. M.; Bott, S. G.; Richmond, M. G. Organometallics 1993, 12, 4779.
Shen, H.; Bott, S. G.; Richmond, M. G. Inorg. Chim. Acta 1996, 250, 195.
Bott, S. G.; Shen, H.; Richmond, M. G. Struct. Chem. 2001, 12, 225.
Hoffmann, R. Angew. Chem. Int. Ed. 1982, 21, 711.
Albright, T. A.; Burdett, J. K.; Whangbo, M. H. Orbital Interactions in Chemistry; Wiley-Interscience: New York, 1985.
Sutin, K. A.; Kolis, J. W.; Mlekuz, M.; Bougeard, P.; Sayer, B. G.; Quilliam, M. A.; Faggiani, R.; Lock, C. J. L.; McGlinchey, M. J.; Jaouen, G. Organometallics 1987, 6, 439.
Sutin, K. A.; Li, L.; Frampton, C. S.; Sayer, B. G.; McGlinchey, M. J. Organometallics 1991, 10, 2362.
Nestle, M. O.; Hallgren, J. E.; Seyferth, D. Inorg. Synth. 1980, 20, 226.
Beurich, H.; Blumhofer, R.; Vahrenkamp, H. Chem. Ber. 1982, 115, 2409.
Blumhofer, R.; Fischer, K.; Vahrenkamp, H. Chem. Ber. 1986, 119, 194.
Shriver, D. F. The Manipulation of Air-Sensitive Compounds; McGraw-Hill: New York, 1969.
Koelle, U. J. Organomet. Chem. 1977, 133, 53.
Albers, M. O.; Coville, N. J. Coord. Chem. Rev. 1984, 53, 227.
Bott, S. G.; Yang, K.; Richmond, M. G. Organometallics 2003, 22, 1383.
Clark, D. T.; Sutin, K. A.; Perrier, R. E.; McGlinchey, M. J. Polyhedron 1988, 7, 2297.
Garrou, P. E. Chem. Rev. 1981, 81, 229.
Richmond, M. G.; Kochi, J. K. Organometallics 1987, 6, 254.
Bott, S. G.; Wang, J. C; Shen, H.; Richmond, M. G. J. Chem. Crystallogr. 1999, 29, 391.
See also: Beurich, H.; Vahrenkamp, H. Angew. Chem. Int. Ed. Engl. 1978, 17, 863.
Orpen, A. G.; Brammer, L.; Allen, F. H.; Kennard, O.; Watson, D. G.; Taylor, R. J. Chem. Soc. Dalton Trans. 1989, SI.
Weast, R. C. (Ed.). Handbook of Chemistry and Physics; CRC Press: Cleveland, OH, 56th ed., 1975.
Duffy, D. N.; Kassis, M. M.; Rae, A. D. J. Organomet. Chem. 1993, 460, 97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bott, S.G., Yang, K., Huang, SH. et al. CO substitution in the mixed-metal clusters PhCCo2Ni(CO)6Cp and PhCCo2Mo(CO)8Cp by (Z)-Ph2PCH=CHPPh2. X-ray diffraction structures and proof of ligand bridging in PhCCo2Ni(CO)4[(Z)-Ph2PCH=CHPPh2]Cp and PhCCo2Mo(CO)6[(Z)-Ph2PCH=CHPPh2]Cp. J Chem Crystallogr 34, 883–891 (2004). https://doi.org/10.1007/s10870-004-7723-3
Issue Date:
DOI: https://doi.org/10.1007/s10870-004-7723-3