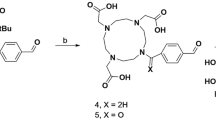
Abstract
Therapeutic embolization of blood vessels is a minimally invasive, catheter-based procedure performed with solid or liquid emboli to treat bleeding, vascular malformations, and vascular tumors. Hepatocellular carcinoma (HCC) affects about half a million people per year. When unresectable, HCC is treated with embolization and local drug therapy by transarterial chemoembolization (TACE). For TACE, drug eluting beads (DC Bead®) may be used to occlude or reduce arterial blood supply and deliver chemotherapeutics locally to the tumor. Although this treatment has been shown to be safe and to improve patient survival, the procedure lacks imaging feedback regarding the location of embolic agent and drug coverage. To address this shortcoming, herein we report the synthesis and characterization of image-able drug eluting beads (iBeads) from the commercial DC Bead® product. Two different radiopaque beads were synthesized. In one approach, embolic beads were conjugated with 2,3,5-triiodobenzyl alcohol in the presence of 1,1′-carbonyldiimidazol to give iBead I. iBead II was synthesized with a similar approach but instead using a trimethylenediamine spacer and 2,3,5-triiodobenzoic acid. Doxorubicin was loaded into the iBeads II using a previously reported method. Size and shape of iBeads were evaluated using an upright microscope and their conspicuity assessed using a clinical CT and micro-CT. Bland and Dox-loaded iBeads II visualized with both clinical CT and microCT. Under microCT, individual bland and Dox loaded beads had a mean attenuation of 7904 ± 804 and 11,873.96 ± 706.12 HU, respectively. These iBeads have the potential to enhance image-guided TACE procedures by providing localization of embolic-particle and drug.








Similar content being viewed by others
References
Chuang VP, Wallace S. Current status of transcatheter management of neoplasms. Cardiovasc Intervent Radiol. 1980;3(4):256–65.
Loffroy R, et al. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7(2):250–63.
Rosch J, Antonovi R, Dotter CT. Selective vasoconstrictor infusion in management of arterio-capillary gastrointestinal hemorrhage. Am J Roentgenol. 1972;116(2):279-&.
Prochask JM, Flye MW, Johnsrud IS. Left gastric artery embolization for control of gastric bleeding—complication. Radiology. 1973;107(3):521–2.
Baum S, Nusbaum M. Control of gastrointestinal hemorrhage by selective mesenteric arterial infusion of vasopressin. Radiology. 1971;98(3):497-&.
Bartling SH, et al. First multimodal embolization particles visible on X-ray/computed tomography and magnetic resonance imaging. Invest Radiol. 2011;46(3):178–86.
Newton TH, Adams JE. Angiographic demonstration and nonsurgical embolization of spinal cord angioma. Radiology. 1968;91(5):873-&.
Luessenh AJ, et al. Clinical evaluation of artificial embolization in management of large cerebral arteriovenous malformations. J Neurosurg. 1965;23(4):400-&.
Chang SD, et al. Multimodality treatment of giant intracranial arteriovenous malformations (Reprinted from Archival article vol 53, pg 1, 2003). Neurosurgery. 2007;61(1):432–42.
Luessenhop AJ, Mujica PH. Embolization of segments of the circle of willis and adjacent branches for management of certain inoperable cerebral arteriovenous-malformations. J Neurosurg. 1981;54(5):573–82.
Doppman JL, Dichiro G, Ommaya AK. Percutaneous embolization of spinal cord arteriovenous malformations. J Neurosurg. 1971;34(1):48-&.
Beaujeux R, et al. Trisacryl gelatin microspheres for therapeutic embolization. 2. Preliminary clinical evaluation in tumors and arteriovenous malformations. Am J Neuroradiol. 1996;17(3):541–8.
Rand T, et al. Arterial embolization of unresectable hepatocellular carcinoma with use of microspheres, lipiodol, and cyanoacrylate. Cardiovasc Intervent Radiol. 2005;28(3):313–8.
Marshburn PB, Matthews ML, Hurst BS. Uterine artery embolization as a treatment option for uterine myomas. Obstet Gynecol Clin North Am. 2006;33(1):125.
Manelfe C, et al. Transfemoral catheter embolization in intracranial meningiomas. Rev Neurol. 1973;128(5):339–51.
Krysl J, Kumpe DA. Embolization agents: a review. Tech Vasc Interv Radiology. 2000;3(3):4.
Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Interv Radiol. 2008;25:12.
Kirchhoff TD, et al. Transarterial chemoembolization using degradable starch microspheres and iodized oil in the treatment of advanced hepatocellular carcinoma: evaluation of tumor response, toxicity, and survival. Hepatobiliary Pancreat Dis Int. 2007;6(3):259–66.
Laurent A, et al. Trisacryl gelatin microspheres for therapeutic embolization. 1. Development and in vitro evaluation. Am J Neuroradiol. 1996;17(3):533–40.
Stampfl S, et al. Biocompatibility and recanalization characteristics of hydrogel microspheres with Polyzene-F as polymer coating. Cardiovasc Intervent Radiol. 2008;31(4):799–806.
Rao VRK, et al. Hydrolyzed microspheres from cross-linked polymethyl methacrylate (hydrogel)—a new embolic material for interventional neuroradiology. J Neuroradiol. 1991;18(1):61–9.
Jayakrishnan A, et al. Hydrogel microspheres from crosslinked poly(methyl methacrylate)—synthesis and biocompatibility studies. Bull Mater Sci. 1989;12(1):17–25.
Ducmauger A, Benoit JP, Puisieux F. Preparation and characterization of cross-linked human-serum albumin microcapsules containing 5-fluorouracil. Pharm Acta Helv. 1986;61(4):119–24.
Benita S, Zouai O, Benoit JP. 5-Fluorouracil - carnauba wax microspheres for chemoembolization—an invitro evaluation. J Pharm Sci. 1986;75(9):847–51.
Spenlehauer G, Veillard M, Benoit JP. Formation and characterization of cisplatin loaded poly(D, L-lactide) microspheres for chemoembolization. J Pharm Sci. 1986;75(8):750–5.
Rodiek SO, Stolzle A, Lumenta CB. Preoperative embolization of intracranial meningiomas with Embosphere((R)) microspheres. Minim Invasive Neurosurg. 2004;47(5):299–305.
Chun HJ, et al. An experimental study for syndiotactic polyvinyl alcohol spheres as an embolic agent: can it maintain spherical shape in vivo? Biomed Mater Eng. 2014;24(4):1743–50.
Lee SG, et al. Preparation of novel syndiotactic poly(vinyl alcohol) microspheres through the low-temperature suspension copolymerization of vinyl pivalate and vinyl acetate and heterogeneous saponification. J Appl Polym Sci. 2005;95(6):1539–48.
Stampfl U, et al. Experimental liver embolization with four different spherical embolic materials: impact on inflammatory tissue and foreign body reaction. Cardiovasc Intervent Radiol. 2009;32(2):303–12.
Malagari K, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008;33(5):512–9.
Grosso M, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an italian multicentre study. Cardiovasc Intervent Radiol. 2008;31(6):1141–9.
Jordan O, et al. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21(7):1084–90.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17.
Forman D, Ferlay J. In: Stewart BW, Wild CP, editors. World cancer report in the global and regional burden of cancer. Lyon Cedex: IARC; 2014. p. 16–53.
Forner A, et al. Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2006;60(2):89–98.
Varela M, et al. Treatment of hepatocellular carcinoma: is there an optimal strategy? Cancer Treat Rev. 2003;29(2):99–104.
Bruix J, et al. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5(3):215–9.
Lin SM. Recent advances of radiofrequency ablation in the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:A74.
Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29(3):285–92.
Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2010;26(3):189–95.
Himoto T, et al. Recent advances in radiofrequency ablation for the management of hepatocellular carcinoma. Hepat Month. 2012;12(10):e5945.
Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: current status. World J Radiol. 2010;2(11):8.
Bruix J, et al. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene. 2006;25(27):3848–56.
Llovet JM, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Sikander A, et al. Transarterial chemoembolization in a patient with recurrent hepatocellular carcinoma and portal vein thrombosis—a case report and review of the literature. Am J Clin Oncol. 2005;28(6):638–9.
O’Toole D, Ruszniewski P. Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19(4):585–94.
Allison DJ, Modlin IM, Jenkins WJ. Treatment of carcinoid liver metastases by hepatic-artery embolization. Lancet. 1977;2(052):1323–5.
Chuang VP, Wallace S, Gianturco C. A new improved coil for tapered-tip catheter for arterial-occlusion. Radiology. 1980;135(2):507–9.
Gianturco C, Anderson JH, Wallace S. Mechanical devices for arterial-occlusion. Am J Roentgenol. 1975;124(3):428–35.
Wallace S, et al. Therapeutic vascular occlusion utilizing steel coil technique—clinical applications. Am J Roentgenol. 1976;127(3):381–7.
Anderson JH, et al. Mini gianturco stainless-steel coils for transcatheter vascular occlusion. Radiology. 1979;132(2):301–3.
Allison DJ, Jordan H, Hennessy O. Therapeutic embolization of the hepatic-artery—a review of 75 procedures. Lancet. 1985;1(8429):595–9.
Llovet JM, Bruix J. Unresectable hepatocellular carcinoma: meta-analysis of arterial embolization. Radiology. 2004;230(1):300–1.
Llovet JM, Bruix J, Barcelona G, Barcelona-Clınic Liver Cancer Group. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–42.
Lo CM, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71.
Dreher MR, et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol. 2012;23(2):257–64.
Lewis AL, et al. Doxorubicin eluting beads-1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18(9):1691–9.
Lewis AL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2):335–42.
Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv. 2011;8(2):153–69.
Lewis AL, Dreher MR. Locoregional drug delivery using image-guided intra-arterial drug eluting bead therapy. J Control Release. 2012;161(2):338–50.
Lewandowski RJ, et al. Transcatheter intraarterial therapies: rationale and overview. Radiology. 2011;259(3):641–57.
Liapi E, Geschwind JFH. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34(1):37–49.
Yamada R, et al. Hepatic-artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148(2):397–401.
Konno T, et al. Effect of arterial administration of high-molecular-weight anti-cancer agent smancs with lipid lymphographic agent on hepatoma—a preliminary-report. Eur J Cancer Clin Oncol. 1983;19(8):1053-&.
Stampfl U, et al. Midterm results of uterine artery embolization using narrow-size calibrated embozene microspheres. Cardiovasc Intervent Radiol. 2011;34(2):295–305.
Sadick M, et al. Application of DC beads in hepatocellular carcinoma: clinical and radiological results of a drug delivery device for transcatheter superselective arterial embolization. Onkologie. 2010;33(1–2):31–7.
Aliberti C, et al. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res. 2011;31(12):4581–7.
Fiorentini G, et al. Intra-arterial hepatic chemoembolization (TACE) of liver metastases from ocular melanoma with slow-release irinotecan-eluting beads. Early results of a phase II clinical study. In Vivo. 2009;23(1):131–7.
Eichler K, et al. First human study in treatment of unresectable liver metastases from colorectal cancer with irinotecan-loaded beads (DEBIRI). Int J Oncol. 2012;41(4):1213–20.
Sharma KV, et al. Development of “imageable” beads for transcatheter embolotherapy. J Vasc Interv Radiol. 2010;21(6):865–76.
Fiorentini G, et al. Trans-arterial chemoembolization of metastatic colorectal carcinoma (MCRC) to the liver adopting polyvinyl alcohol microspheres (PAM) loaded with irinotecan compared with folfiri (CT): evaluation at two years of a phase III clinical trial. Ann Oncol. 2010;21:191.
Gonzalez MV, et al. Doxorubicin eluting beads—2: methods for evaluating drug elution and in vitro: in vivo correlation. J Mater Sci Mater Med. 2008;19(2):767–75.
Tang Y, et al. Evaluation of irinotecan drug-eluting beads: a new drug-device combination product for the chemoembolization of hepatic metastases. J Control Release. 2006;116(2):E55–6.
Taylor RR, et al. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci. 2007;30(1):7–14.
Kaiser E, et al. Color test for detection of free terminal amino groups in solid-phase synthesis of peptides. Anal Biochem. 1970;34(2):595-&.
Mawad D, et al. Synthesis and characterization of radiopaque iodine-containing degradable PVA hydrogels. Biomacromolecules. 2008;9(1):263–8.
Okamura M, et al. Synthesis and properties of radiopaque polymer hydrogels: polyion complexes of copolymers of acrylamide derivatives having triiodophenyl and carboxyl groups and p-styrene sulfonate and polyallylamine. J Mol Struct. 2000;554(1):35–45.
Jayakrishnan A, et al. Preparation and evaluation of radiopaque hydrogel microspheres based on phema iothalamic acid and phema iopanoic acid as particulate emboli. J Biomed Mater Res. 1990;24(8):993–1004.
Bendszus M, et al. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. Am J Neuroradiol. 2000;21(2):255–61.
Bendszus M, et al. MR imaging- and MR spectroscopy-revealed changes in meningiomas for which embolization was performed without subsequent surgery. Am J Neuroradiol. 2000;21(4):666–9.
Gaba RC, et al. Ethiodized oil uptake does not predict doxorubicin drug delivery after chemoembolization in VX2 liver tumors. J Vasc Interv Radiol. 2012;23(2):265–73.
Okamura M, et al. Synthesis and properties of radiopaque polymer hydrogels II: copolymers of 2,4,6-triiodophenyl- or N-(3-carboxy-2,4,6-triiodophenyl)-acrylamide and p-styrene sulfonate. J Mol Struct. 2002;602:17–28.
Thanoo BC, Jayakrishnan A. Barium sulfate-loaded P(HEMA) microspheres as artificial emboli—preparation and properties. Biomaterials. 1990;11(7):477–81.
Jayakrishnan A, Knepp WA, Goldberg EP. Casein microspheres—preparation and evaluation as a carrier for controlled drug-delivery. Int J Pharm. 1994;106(3):221–8.
Thanoo BC, Sunny MC, Jayakrishnan A. Tantalum-loaded polyurethane microspheres for particulate embolization—preparation and properties. Biomaterials. 1991;12(5):525–8.
Horak D, et al. Hydrogels in endovascular embolization. 3. radiopaque spherical-particles, their preparation and properties. Biomaterials. 1987;8(2):142–5.
Thanoo BC, Jayakrishnan A. Radiopaque hydrogel microspheres. J Microencapsul. 1989;6(2):233–44.
Hagit A, et al. Synthesis and characterization of dual modality (CT/MRI) core-shell microparticles for embolization purposes. Biomacromolecules. 2010;11(6):1600–7.
Acknowledgments
This research was supported by the Center for Interventional Oncology in the Intramural Research Program of the National Institutes of Health (NIH). NIH and Biocompatibles UK Ltd, a BTG International group company have a Cooperative Research and Development Agreement. C.G.J. was supported by the Imaging Sciences Training Program of the National Institutes of Health. We would like to thank Belhu Metaferia, for his advice and useful discussions.
Conflict of interest
Ayele H. Negussie, Carmen Gacchina Johnson, Gert Storm, Karun V. Sharma and Bradford J. Wood do not have any forms of conflicts of interest. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the National Institutes of Health. The authors alone are responsible for the content and opinions of the paper. NIH and Biocompatibles UK Ltd, a BTG International group company have a cooperative research and development agreement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negussie, A.H., Dreher, M.R., Johnson, C.G. et al. Synthesis and characterization of image-able polyvinyl alcohol microspheres for image-guided chemoembolization. J Mater Sci: Mater Med 26, 198 (2015). https://doi.org/10.1007/s10856-015-5530-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5530-3