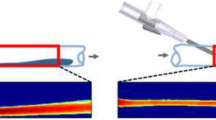
Abstract
Infusate backflow or leak-back along the cannula track can occur during intraparenchymal infusions resulting in non-specific targeting of therapeutic agents. The occurrence of backflow depends on several variables including cannula radius, infusate flow rate, and tip location. In this study, polymer coatings that swell in situ were developed and tested with in vitro hydrogel experiments for backflow reduction. Coatings were applied to the external cannula surface in a dual layer arrangement with a poly(vinyl alcohol) outer layer atop an inner poly(ethylene oxide) and alginate layer. Once these coated cannulas were inserted and allotted an 8–10 min waiting period for hydration, backflow during infusions of 4.0 μl of a macromolecular tracer (Evans Blue labeled albumin) was reduced significantly under flow rates of 0.3–0.6 μl/min, allowing for more effective distribution within targeted regions. Polymer coating thicknesses before and after hydrations were 0.035 and 0.370 mm, respectively. Also, backflow data was fit to a model to estimate the effective local compressive stress caused by the hydrated polymers. After withdrawal of the cannula from the insertion site, the hydrated polymer coatings remained within the cavity left in the hydrogel tissue phantom and formed a seal at the infusion site that prevented further backflow during needle withdrawal. Ex vivo infusions in excised porcine brain tissues also showed significant backflow reduction while also demonstrating the ability to leave a polymer seal in the tissue cavity after cannula removal. Thus, application of these polymers as needle or cannula coatings offers a potentially simple method to improve targeting for local drug delivery.







Similar content being viewed by others
References
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–80.
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–95.
Rogawski MA. Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics. 2009;6(2):344–51.
Sampson JH, Brady ML, Petry NA, Croteau D, Friedman AH, Friedman HS, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60(2):89–98.
Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90(2):315–20.
Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187(1):46–51.
Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics (vol 277, pg R1218, 1999). Am J Physiol Regul Integr Comp Physiol. 2002;282(6):R1218–R1229.
Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Control Release. 2006;111(3):252–62.
Guarnieri M, Carson BS, Khan A, Penno M, Jallo GI. Flexible versus rigid catheters for chronic administration of exogenous agents into central nervous system tissues. J Neurosci Methods. 2005;144(2):147–52.
Jagannathan J, Walbridge S, Butman JA, Oldfield EH, Lonser RR. Effect of ependymal and pial surfaces on convection-enhanced delivery. Laboratory investigation. J Neurosurg. 2008;109(3):547–52.
Cho MH, Malhotra A, Donahue DM, Wain JC, Harris RS, Karmpaliotis D, et al. Mechanical ventilation and air leaks after lung biopsy for acute respiratory distress syndrome. Ann Thorac Surg. 2006;82(1):261–7.
Kelley ML, Mosenthal WT, Milne J. Bile leakage following menghini needle liver biopsy. J Am Med Assoc. 1971;216(2):333–333(1).
Wiksell H, Schassburger KU, Janicijevic M, Leifland K, Lofgren L, Rotstein S, et al. Prevention of tumour cell dissemination in diagnostic needle procedures. Br J Cancer. 2010;103(11):1706–9.
Bjugstad KB, Lampe K, Kern DS, Mahoney M. Biocompatibility of poly(ethylene glycol)-based hydrogels in the brain: an analysis of the glial response across space and time. J Biomed Mater Res A. 2010;95A(1):79–91.
Jiang YJ, Schadlich A, Amado E, Weis C, Odermatt E, Mader K, et al. In vivo studies on intraperitoneally administrated poly(vinyl alcohol). J Biomed Mater Res B. 2010;93B(1):275–84.
Chen ZJ, Gillies GT, Broaddus WC, Prabhu SS, Fillmore H, Mitchell RM, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. J Neurosurg. 2004;101(2):314–22.
Felix B, Leger ME, Albe-Fessard D. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49(1–2):1–137.
Raghavan R, Mikaelian S, Brady M, Chen ZJ. Fluid infusions from catheters into elastic tissue: I. Azimuthally symmetric backflow in homogeneous media. Phys Med Biol. 2010;55(1):281–304.
Chen XM, Astary GW, Sepulveda H, Mareci TH, Sarntinoranont M. Quantitative assessment of macromolecular concentration during direct infusion into an agarose hydrogel phantom using contrast-enhanced MRI. Magn Reson Imaging. 2008;26(10):1433–41.
Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–10.
Soonshiong P, Feldman E, Nelson R, Heintz R, Yao Q, Yao ZW, et al. Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci USA. 1993;90(12):5843–7.
Soonshiong P, Heintz RE, Merideth N, Yao QX, Yao ZW, Zheng TL, et al. Insulin independence in a type-1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343(8903):950–1.
Mirshafiey A, Borzooy Z, Abhari RS, Razavi A, Tavangar M, Rehm BHA. Treatment of experimental immune complex glomerulonephritis by sodium alginate. Vasc Pharmacol. 2005;43(1):30–5.
Lavrov VP. The experimental treatment of acute edema of the brain with sodium alginate From: Ref Zh Otd Vypusk Farmakol Toksikol, 1964, no. 22.54.98 (translation). Sb Nauch Tr Vladi Vostokskh Med Inst. 1964;2(1):9–10.
Hagen A, Skjak-Braek G, Dornish M. Pharmacokinetics of sodium alginate in mice. Eur J Pharm Sci. 1996;4(Suppl):100–100(1).
Solandt OM. Some observations upon sodium alginate. Q J Exp Physiol Cogn Med Sci. 1942;31:25–30.
Chenoweth MB. The toxicity of sodium alginate in cats. Ann Surg. 1948;127(6):1173–81.
Smyth HF, Weil CS, Woodside MD, Knaak JB, Sullivan LJ, Carpente CP. Experimental toxicity of a high molecular weight poly(ethylene oxide). Toxicol Appl Pharmacol. 1970;16(2):442–445.
Fruijtier-Polloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005;214(1–2):1–38.
Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000;14(1):27–35.
Shi R, Borgens RB. Acute repair of crushed guinea pig spinal cord by polyethylene glycol. J Neurophysiol. 1999;81(5):2406–14.
Shi R, Borgens RB. Anatomical repair of nerve membranes in crushed mammalian spinal cord with polyethylene glycol. J Neurocytol. 2000;29(9):633–43.
Smucker P, Hekmatyar SK, Bansal N, Rodgers RB, Shapiro SA, Borgens RB. Intravenous polyethylene glycol successfully treats severe acceleration-induced brain injury in rats as assessed by magnetic resonance imaging. Neurosurgery. 2009;64(5):984–90.
Tabata T, Murakami Y, Ikada Y. Tumor accumulation of poly(vinyl alcohol) of different sizes after intravenous injection. J Control Release. 1998;50(1–3):123–33.
Jiang YJ, Schadlich A, Amado E, Weis C, Odermatt E, Mader K, et al. In vivo studies on intraperitoneally administrated poly(vinyl alcohol). J Biomed Mater Res B. 2010;93B(1):275–84.
Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21(5):207–15.
Acknowledgments
We would like to thank Ronja Månsson for her preliminary work on this project as well as Gregory Pishko and Benjamin Oberstein for their assistance in the construction of the current experimental infusion setup. The project described was supported by award number R01NS063360 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Neurological Disorders and Stroke or the National Institutes of Health. This work was also supported in part by NIH/NCRR CTSA award to the University of Florida UL1 RR029890, as well as the University Scholars Program, University of Florida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vazquez, L.C., Hagel, E., Willenberg, B.J. et al. Polymer-coated cannulas for the reduction of backflow during intraparenchymal infusions. J Mater Sci: Mater Med 23, 2037–2046 (2012). https://doi.org/10.1007/s10856-012-4652-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4652-0