Abstract
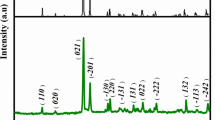
Polymorphs of MnO2 are known to have different electrochemical activity, with gamma (γ) and akhtenskite (ε) polymorphs often considered as the most active phases for aqueous battery cathodes. However, most synthetic samples contain a mixture of polymorph phases, which makes understanding of the structure-property correlations more complicated. In this paper, we report on a systematic study that correlates synthesis parameters with the morphology, phase composition and reversible storage capacity of the resulting nanoparticles. Rietveld analysis of X-ray powder diffraction patterns was used to accurately describe fractional composition of multi-phase nanoparticles. It was demonstrated that through control of the synthesis parameters desired phase compositions and nanoparticle morphologies can be achieved. The key synthesis parameters were found to be the concentration of Mn2+ precursor which strongly affects both the morphology and the crystalline structure of the products, and the time of reaction. The presence of surfactant only impacts the crystalline phase composition of the MnO2 nanoparticles and has insignificant effect on the morphology. It was also demonstrated that nanoparticles with higher fraction of the akhtenskite polymorph show higher reversible capacities in LiOH electrolyte (~210 mAh/g) compared to other MnO2 phase compositions (~120 mAh/g).





Similar content being viewed by others
References
Liang S, Teng F, Bulgan G, Zong R, Zhu Y (2008) Effect Of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J Phys Chem C 112:5307–5315
Zhang Q, Li S, Sun S, Yin X, Yu J (2009) Lithium selective adsorption on 1-D MnO2 nanostructure ion-sieve. Adv Powder Technol 20:432–437
Xu J, Zhao W, Luo X, Chen H (2005) A sensitive biosensor for lactate based on layer-by-layer assembling Mno2 nanoparticles and lactate oxidase on ion-sensitive field-effect transistors. Chem Commun 6:792–794
Liu R, Lee S (2008) MnO2/poly(3,4-ethylenedioxythiophene) coaxial nanowires by one-step coelectrodeposition for electrochemical energy storage. J Am Chem Soc 130:2942–2943
Li Q, Wang Z, Li G, Guo R, Ding L, Tong Y (2012) Design and synthesis of MnO2/Mn/MnO2 sandwich-structured nanotube arrays with high supercapacitive performance for electrochemical energy storage. Nano Lett 12:3803–3807
Cheng F, Chen J, Gou X, Shen P (2005) High-power alkaline Zn-MnO2 batteries using Γ-MnO2 nanowires/nanotubes and electrolytic zinc powder. Adv Mater 17:2753–2756
Linden D, Reddy T (2002) Handbook of batteries. McGraw-Hill, New York
Einerhand REF, Visscher W (1991) Zinc electrode shape change. J Electrochem Soc 138:7–17
Bai L, Qu DY, Conway BE, Zhou YH, Chawdhury G, Adams WA (1993) Rechargeability of a chemically modified MnO2Zn battery system at practically favorable power levels. J Electrochem Soc 140(4):884–889
Wolfenstine J, Foster D, Behl W, Gilman S (1998) Gas evolution and self-discharge in Li/MnO2 primary batteries. Army Research Laboratory, Adelphi
Wang X, Li Y (2003) Synthesis and formation mechanism of manganese dioxide nanowires/nanorods. Chem Eur J 9(1):300–306
Hill LI, Verbaere A, Guyomard D (2002) Synthesis of alpha-, beta-, and low defect gamma-manganese dioxides using the electrochemical-hydrothermal method and study of their Li insertion behavior. J New Mater Electrochem Syst 5(2):129–134
Vodyanitskii YN (2004) Formation of manganese oxides in soils. Eurasian Soil Sci C/C Pochvovedenie 37(6):572–584
Ching S, Petrovay DJ, Jorgensen ML, Suib SL (1997) Sol–gel synthesis of layered birnessite-type manganese oxides. Inorg Chem 36(5):883–890
Ding Y-S, Shen X-F, Gomez S, Luo H, Aindow M, Suib SL (2006) Hydrothermal growth of manganese dioxide into three-dimensional hierarchical nanoarchitectures. Adv Funct Mater 16(4):549–555
De Wolff PM (1959) Interpretation of some γ-MnO2 Diffraction patterns. Acta Crystallogr A 12(4):341–345
Lv D, Huang X, Yue H, Yang Y (2009) Sodium-ion-assisted hydrothermal synthesis of γ-MnO2 and Its electrochemical performance. J Electrochem Soc 156(11):A911–A916
Devaraj S, Munichandraiah N (2008) Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112(11):4406–4417
Liu X, Chen C, Zhao Y, Jia B (2013) A review on the synthesis of manganese oxide nanomaterials and their applications on lithium-ion batteries. J Nanomater 2013:1–7
Rus ED, Moon GD, Bai J, Steingart DA, Erdonmez CK (2016) Electrochemical behavior of electrolytic manganese dioxide in aqueous KOH and LiOH solutions: a comparative study. J Electrochem Soc 163(3):A356–A363
Manickam M, Singh P, Issa TB, Thurgate S, De Marco R (2004) Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery. J Power Sources 130(1–2):254–259
Ruetschi P (1984) Cation-vacancy model for MnO2. J Electrochem Soc 131(12):2737–2744
Guo Y-G, Hu J-S, Wan L-J (2008) Nanostructured materials for electrochemical energy conversion and storage devices. Adv Mater 20(15):2878–2887
Timofeeva EV, Routbort JL, Singh D (2009) Particle shape effects on thermophysical properties of alumina nanofluids. J Appl Phys 106(1):014304
Cheng F, Zhao J, Song W, Li C, Ma H, Chen J, Shen P (2006) Facile controlled synthesis of MnO2 nanostructures of novel shapes and their application in batteries. Inorg Chem 45(5):2038–2044
Subramanian V, Zhu H, Vajtai R, Ajayan PM, Wei B (2005) Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J Phys Chem B 109(43):20207–20214
Wang X, Li Y (2002) Selected-control hydrothermal synthesis of α- and β- MnO2 single crystal nanowires. J Am Chem Soc 124(12):2880–2881
Xiao W, Xia H, Fuh JYH, Lu L (2009) Growth of single-crystal α-MnO2 nanotubes prepared by a hydrothermal route and their electrochemical properties. J Power Sources 193(2):935–938
Thanh NTK, Maclean N, Mahiddine S (2014) Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev 114(15):7610–7630
Lin M, Fu ZY, Tan HR, Tan JPY, Ng SC, Teo E (2012) Hydrothermal synthesis of CeO2 nanocrystals: ostwald ripening or oriented attachment? Cryst Growth Des 12(6):3296–3303
Pacholski C, Kornowski A, Weller H (2002) Self-assembly of ZnO: from nanodots to nanorods. Angew Chem Int Ed 41(7):1188–1191
Lee EJH, Ribeiro C, Longo E, Leite ER (2005) Oriented attachment: an effective mechanism in the formation of anisotropic nanocrystals. J Phys Chem B 109(44):20842–20846
Wang EI, Lin L, Bowden WL (1996) Electrochemical cell comprising gamma MnO2 cathode having filamentary protrusions. J Power Sources 63(2):294
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2(2):65–71
Larson AC, Von Dreele RB (2000) General structure analysis system (GSAS); Report LAUR 86-748. Los Alamos, NM, Los Alamos National Laboratory
Toby BH (2001) EXPGUI, a graphical user interface for GSAS. J Appl Crystallogr 34(2):210–213
Karlsruhe F. ICSD https://icsd.fiz-karlsruhe.de/search/index.xhtml. Accessed 21 Oct 2016
Peng X, Manna L, Yang W, Wickham J, Scher E, Kadavanich A, Alivisatos AP (2000) Shape control of CdSe nanocrystals. Nature 404(6773):59–61
Shukla N, Nigra MM, Nuhfer T, Bartel MA, Gellman AJ (2009) Tailoring the shapes of Fe X Pt 100−X nanoparticles. Nanotechnology 20(6):065602
Kumar S, Gradzielski M, Mehta SK (2013) The critical role of surfactants towards CdS nanoparticles: synthesis, stability, optical and PL emission properties. RSC Adv 3(8):2662–2676
Petroski JM, Wang ZL, Green TC, El-Sayed MA (1998) kinetically controlled growth and shape formation mechanism of platinum nanoparticles. J Phys Chem B 102:3316–3320
Ahmadi TS, Wang ZL, Green TC, Henglein A, El-Sayed MA (1996) Shape-controlled synthesis of colloidal platinum nanoparticles. Science 272(5270):1924–1926
Jana NR, Gearheart L, Murphy CJ (2001) Seed-mediated growth approach for shape-controlled synthesis of spheroidal and rod-like gold nanoparticles using a surfactant template. Adv Mater 13(18):1389–1393
Xu W, Lan H, Wang H, Liu H, Qu J (2015) Comparing the adsorption behaviors of Cd, Cu and Pb from Water onto Fe–Mn binary oxide, MnO2 and FeOOH. Front Environ Sci Eng 9(3):385–393
Hertzberg BJ, Huang A, Hsieh A, Chamoun M, Davies G, Seo JK, Zhong Z, Croft M, Erdonmez C, Meng YS, Steingart D (2016) Effect of multiple cation electrolyte mixtures on rechargeable Zn–MnO2 alkaline battery. Chem Mater 28(13):4536–4545
Minakshi M, Singh P, Issa TB, Thurgate S, Marcob RD (2004) Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery Part I. A preliminary study. J Power Sources 130:254–259
Malloy AP, Browning GJ, Donne SW (2005) Surface characterization of heat-treated electrolytic manganese dioxide. J Colloid Interface Sci 285(2):653–664
Acknowledgements
We thank Dr. James Kaduk for helpful discussions on XRD analysis. This research was funded by US Department of Energy, Advanced Research Funding Agency—Energy (ARPA-E) (award #AR000387). Use of the Argonne National Laboratory, Center for Nanoscale Materials and Electron Microscopy Center are supported by the US Department of Energy, under Contract No. DE-AC02- 06CH11357.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moazzen, E., Timofeeva, E.V. & Segre, C.U. Controlled synthesis of MnO2 nanoparticles for aqueous battery cathodes: polymorphism–capacity correlation. J Mater Sci 52, 8107–8118 (2017). https://doi.org/10.1007/s10853-017-1018-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1018-5