Abstract
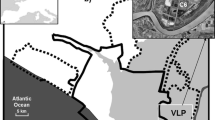
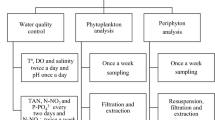
Phytoplankton community composition, density, and succession were studied in tropical commercial ponds with euryhaline tiger shrimp (Penaeus monodon Fabricius) using green-water technology at two different stocking densities [T1 10 post-larvae (PL) m−2 and T2 15 PL m−2] in one grow-out season (May–October 2005) in Leganes, Iloilo, Philippines. Weekly qualitative and quantitative analyses of phytoplankton were done along with physicochemical analyses of the pond waters. A total of 103 taxa belonging to nine different algal classes were observed. Of these classes, the Chlorophyceae, Cyanophyceae and Bacillariophyceae constituted the great bulk of the phytoplankton population. The two treatments did not show any significant differences in the growth pattern of phytoplankton over time and in their diversity indices. Although T2 had higher values than T1 for algal density and species diversity index, the differences were not significant. The mean Shannon-Wiener diversity index for T2 (1.56) was higher than T1 (1.39) but not significantly different. Both treatment ponds had Chlorophyceae as the dominant algae during the initial culture phase [0–35 days of culture (DOC)], which coincided with high salinity (average = 35.67 ppt) and relatively high N:P ratios (average = 1.95). The chlorophycean bloom was made up mostly of Nannochloropsis sp. The cyanophycean bloom occurred towards the final culture phase (84–112/126 DOC) when there was low salinity (average = 19.5 ppt) and relatively high N:P ratios (average 2.01). A short diatom bloom occurred in T2 at the same time that the N:P ratios rose dramatically to 4.2 at 42 DOC. Among the eight physicochemical parameters examined, positive correlations were noted among alkalinity, ammonium-nitrogen, nitrite-nitrogen and phytoplankton community. High species diversity index and species richness could have enhanced the stability of favorable Nannochloropsis blooms, especially in T2. No differences were noted between the two treatments in terms of the shrimp’s biomass at harvest time (T1 = 28.9 and T2 = 29.4 g fresh wt per shrimp), although a significantly higher survival rate (P < 0.05) was observed in T1 (97%) than in T2 (56%). Both treatments were able to control the occurrence of the luminous bacterium Vibrio harveyi.




Similar content being viewed by others
References
Alonso-Rodriguez R, Paez-Osuna F (2003) Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: a review with special reference to the situation in the Gulf of California. Aquaculture 219:317–336
Apud FD (1985) Extensive and semi-intensive culture of prawn and shrimp in the Philippines. In: Taki Y, Primavera JH, Llubrera IA (eds) Proceedings of the first international conference on the culture of penaeid prawns/shrimps. Southeast Asian Fisheries Development Centre, Iloilo City, Philippines, pp. 105–113
Barica J (1975) Collapses of algal blooms in prairie potholes lakes: their mechanism and ecological impact. Verh Int Verein Limnol 19:606–615
Boyd CE (1979) Water quality in warm water fish ponds. Alabama Agriculture Experiment Station, Auburn University, Auburn, AL, p 482
Burford M (1997) Phytoplankton dynamics in shrimp ponds. Aquacult Res 28:351–360
Buttino I (1994) The effect of low concentrations of phenol and ammonia on egg production rates, fecal pellet production and egg viability of the calanoid copepod Acartia clause. Mar Biol 119:629–634
Chien YH (1992) Water quality requirements and management for marine shrimp culture. In: Wyban J (ed) Proceedings of the special session on shrimp farming. World Aquaculture Society, Baton Rouge, pp 30–42
Clifford HC (1992) Marine shrimp pond management: a review. In: Wyban J (ed) Proceedings of the special session on shrimp farming. World Aquaculture Society, Baton Rouge, pp 100–137
Corre VL Jr, Janeo RL, Caipang CMA, Calpe AT (1999) Sustainable shrimp culture techniques: use of probiotics and reservoirs with “green water.” Philippine Council for Aquatic and Marine Research and Development, Los Baños, Laguna, and University of the Philippines Visayas, Miag-ao, Iloilo 32p
Corre VL Jr, Janeo RL, Ronquillo JD, Kurokura H (2005) Use of greenwater technology as biocontrol of luminous bacteria in intensive shrimp (Penaeus monodon) grow-out culture. UPV J Nat Sci 10(1):51–60
Kuljis AM, Brown CL (1992) A market study of specific pathogen-free shrimp. Publ no. 112. Center for Tropical and Subtropical Aquaculture, Waimanalo, HI, p 54
Margalef R (1978) Diversity. In: Sournia S (ed) Phytoplankton manual: monographs on oceanographic methodology. Page Brothers, Norwich, UK, pp 251–260
Martinez MR, Chakroff RP, Pantastico JB (1975) Direct phytoplankton counting technique using the haemacytometer. Phil Agr 55:43–50
Odum E (1971) Fundamentals of ecology. Saunders, Philadelphia, p 574
Paerl HW (1988) Nuisance phytoplankton blooms in coastal, estuarine and inland waters. Limnol Oceanogr 33:823–847
Paerl HW, Tucker CS (1995) Ecology of blue-green algae in aquaculture ponds. J World Aquacult Soc 26(2):109–131
Patrick R, Reimer CW (1966) The diatoms of the United States exclusive of Alaska and Hawaii. Vol. 1. Monograph no. 13. Academy of Natural Sciences, Philadelphia, p 688
Prescott GW (1962) Algae of the Western Great Lakes area with an illustrated key to the genera of desmids and freshwater diatoms. Brown, Dubuque, IA, p 977
Primavera JH (1992) Prawn/shrimp culture in the Philippines. In: Fast AQ, Lester LJ (eds) Marine shrimp culture: principles and practices. Elsevier, Amsterdam, pp 701–728
Primavera JH, Apud F, Usigan S (1976) Effect of different stocking densities on survival and growth of sugpo (Penaeus monodon Fabricius) in milkfish-rearing ponds. Phil J Sci 105:193–203
Round FE, Crawford RM, Mann DG (1990) The diatoms: biology and morphology of the genera. Cambridge University Press, New York, p 747
Smith VH (1983) Low nitrogen to phosphorus ratio favors dominance by blue-green algae in lake phytoplankton. Science 221:669–671
Smith DW (1985) Biological control of excessive phytoplankton growth and the enhancement of aquacultural production. Can J Fish Aquat Sci 42:1940–1945
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bulletin 167. Fisheries Research Board of Canada, Ottawa, p 310
Sullivan BK, Ritacco PJ (1985) Ammonia toxicity to larval copepods in eutrophic marine ecosystems: a comparison of results from bioassays and enclosed experimental ecosystems. Aquatic Toxicol 7:2005–2217
Tacon AJ (2003) Aquaculture production trends analysis. In: FAO Inland Water Resources and Aquaculture Service (ed) Review of the state of world aquaculture. FAO Fisheries circular no. 886, rev. 2. FAO, Rome, pp 5–29
Tomas CR (1997) Identifying marine phytoplankton. Academic Press, London, p 858
Vanni MJ, Findlay DL (1990) Trophic cascades and phytoplankton community structure. Ecology 71:921–937
Wang JQ, Li D, Dong S, Wang K, Tian X (1998) Experimental studies on polyculture in closed shrimp ponds I. Intensive polyculture of Chinese shrimp (Penaeus chinensis) with tilapia hybrids. Aquaculture 163:11–27
Yap WG (2000) Shrimp culture: a global overview. Bay Bengal News 2(16):5–12
Yusoff YM, Zubaidah MS, Matias HB, Kwan TS (2002) Phytoplankton succession in intensive marine shrimp culture ponds with a commercial bacterial product. Aquacult Res 33:269–278
Ziemann DA, Walsh WA, Saphore EG, Fulton-Bennett K (1992) A survey of water quality characteristics of effluent from Hawaiian aquaculture facilities. J World Aquacult Soc 23:180–191
Acknowledgements
The authors would like to thank the research staff of the Brackishwater Aquaculture Center (BAC), University of the Philippines Visayas, College of Fisheries in Leganes, Iloilo, Philippines, for all the support provided during the thesis research of the senior author. We also thank Dr. C.M. Caipang and Dr. J.D. Ronquillo for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cremen, M.C.M., Martinez-Goss, M.R., Corre, V.L. et al. Phytoplankton bloom in commercial shrimp ponds using green-water technology. J Appl Phycol 19, 615–624 (2007). https://doi.org/10.1007/s10811-007-9210-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9210-7