Abstract
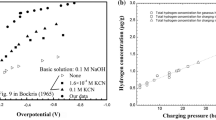
Sacrificial coatings, such as Zn and Cd, are used to protect steel against corrosion. During the electrodeposition of metals, hydrogen is evolved due to electrolysis. The evolved hydrogen may diffuse outward and become trapped in the substrate/coating interface or migrate inward into the steel lattice causing delayed embrittlement when the component is subjected to stress. This study reports two principal variables for Zn, Zn–Ni, and Cd coatings: (i) the quantity of hydrogen absorbed by the coating and substrate by vacuum thermal desorption and (ii) the permeability of the coating material to hydrogen by electrochemical permeation. The findings were analyzed in correlation with the microstructural characteristics of both the coating material and the coating/substrate interface. With Zn–Ni, both coating process and coating material combined to significantly reduce the risk of internal hydrogen embrittlement by (i) introducing the least amount of hydrogen during the electrodeposition process and (ii) by the ease with which hydrogen can be extracted by baking due to the presence of cracks in the coating.












Similar content being viewed by others
References
Oriani RA (1978) Hydrogen embrittlement of steels. Annu Rev Mater Sci 8(1):327–357. doi:10.1146/annurev.ms.08.080178.001551
Oriani RA, Hirth JP, Smialowski M (1985) Hydrogen degradation of ferrous alloys. William Andrew Publishing, Noyes
Brahimi S, and Yue S (2009) Effect of surface processing variables and coating characteristics on hydrogen embrittlement of steel fasteners part 2: electroplating and non electrolytic processes. In: Louisville KY (ed.) SUR/FIN, NASF, 16 June 2009
Coleman DH, Popov BN, White RE (1998) Hydrogen permeation inhibition by thin layer Zn–Ni alloy electrodeposition. J Appl Electrochem 28(9):889–894. doi:10.1023/A:1003408230951
Figueroa D, Robinson MJ (2008) The effects of sacrificial coatings on hydrogen embrittlement and re-embrittlement of ultra high strength steels. Corros Sci 50(4):1066–1079. doi:10.1016/j.corsci.2007.11.023
Hillier EMK, Robinson MJ (2004) Hydrogen embrittlement of high strength steel electroplated with zinc-cobalt alloys. Corros Sci 46(3):715–727. doi:10.1016/S0010-938x(03)00180-X
Hillier EMK, Robinson MJ (2006) Permeation measurements to study hydrogen uptake by steel electroplated with zinc-cobalt alloys. Corros Sci 48(5):1019–1035. doi:10.1016/j.corsci.2005.05.009
ASTM-F1941-00 (2006) F1941-00 Standard specification for electrodeposited coatings on threaded fasteners (Unified Inch Screw Threads (UN/UNR)). ASTM International, West Conshohocken, pp 19428–2959. www.astm.org. doi:10.1520/F1941-07
ASTM-F519-10 (2010) F519-10, Standard test method for mechanical hydrogen embrittlement evaluation of plating/coating processes and service environments. ASTM International, West Conshohocken. www.astm.org. doi:10.1520/F0519-10
MIL-STD-870C (2009) U.S. military standard (USAF) on cadmium plating, low embrittlement, electrodeposition. Department of defence standard practice
Turnbull A, Hutchings RB, Ferriss DH (1997) Modelling of thermal desorption of hydrogen from metals. Mater Sci Eng 238(2):317–328. doi:10.1016/S0921-5093(97)00426-7
Addach H, Bercot P, Wery M, Rezrazi M (2004) Quantitative determination of hydrogen in solids by gas chromatography. J Chromatogr A 1057(1–2):219–223. doi:10.1016/j.chroma.2004.09.067
ASTM-G148-97 (2011) G148-97 Standard practice for evaluation of hydrogen uptake, permeation, and transport in metals by an electrochemical Technique. ASTM International, West Conshohocken. www.astm.org. doi:10.1520/G0148-97R11
Devanathan MAV, Stachurski Z (1962) The adsorption and diffusion of electrolytic hydrogen in palladium. Proc R Soc Lond A 270(1340):90–102. doi:10.1098/rspa.1962.0205
Thomas BK, Fray DJ (1981) The effect of additives on the morphology of zinc electrodeposited from a zinc-chloride electrolyte at high-current densities. J Appl Electrochem 11(6):677–683. doi:10.1007/bf00615170
Bories C, Bonino JP, Rousset A (1999) Structure and thermal stability of zinc-nickel electrodeposits. J Appl Electrochem 29(9):1045–1051. doi:10.1023/a:1003574625112
Brooks I, Erb U (2001) Hardness of electrodeposited microcrystalline and nanocrystalline gamma-phase Zn–Ni alloys. Scripta Mater 44(5):853–858. doi:10.1016/s1359-6462(00)00680-1
Bruet-Hotellaz, Bonino JP, Rousset A, Marolleau, Chauveau E (1999) Structure of zinc-nickel alloy electrodeposits. J Mater Sci 34(4):881–886. doi:10.1023/a:1004553803788
Morón LE, Méndez A, Castañeda F, Flores JG, Ortiz-Frade L, Meas Y, Trejo G (2011) Electrodeposition and corrosion behavior of Zn coatings formed using as brighteners arene additives of different structure. Surf Coat Technol 205(21–22):4985–4992. doi:10.1016/j.surfcoat.2011.04.090
Abdelhalim AM, Baghlaf AO, Sobahi MI (1984) Effect of some addition agents on the electrodeposition of cadmium from acidic chloride baths. Surf Technol 22(2):129–142. doi:10.1016/0376-4583(84)90049-9
Elhalim AMA, Sobahi MI (1983) Effect of bath constituents and some plating variables on the electrodeposition of cadmium from acidic chloride baths. Surf Technol 19(1):45–57. doi:10.1016/0376-4583(83)90018-3
Lin C, Lee H, Hsieh S (1999) Microcracking of flash coatings and its effect on the Zn–Ni coating adhesion of electrodeposited sheet steel. Metall Mate Trans A 30(2):437–448. doi:10.1007/s11661-999-0333-0
Sasaki T, Hirose Y (1994) Residual stress distribution in electroplated Zn–Ni alloy layer determined by X-ray diffraction. Thin Solid Films 253(1–2):356–361. doi:10.1016/0040-6090(94)90347-6
Boes N, Züchner H (1976) Electrochemical methods for studying diffusion, permeation and solubility of hydrogen in metals. J Less Common Metals 49:223–240. doi:10.1016/0022-5088(76)90037-0
Cao Y (2002) Influence of structure of palladium and nickel based membranes on hydrogen permeation. Thesis (Ph D), McGill University, Montreal, Quebec, Canada
Early JG (1978) Hydrogen diffusion in palladium by galvanostatic charging. Acta Metall 26(8):1215–1223. doi:10.1016/0001-6160(78)90005-6
Brahimi S (2007) Effect of surface processing variables on hydrogen embrittlement of steel fasteners. Thesis (M Eng), Mcgill University, Montreal, Quebec, Canada
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sriraman, K.R., Brahimi, S., Szpunar, J.A. et al. Hydrogen embrittlement of Zn-, Zn–Ni-, and Cd-coated high strength steel. J Appl Electrochem 43, 441–451 (2013). https://doi.org/10.1007/s10800-013-0529-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0529-2