Abstract
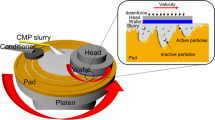
Conventional copper chemical mechanical planarization (CMP) techniques are being pushed to their limits by increasing industrial standards caused by device miniaturization and the use of new materials. There is a need to investigate alternative methods of polishing to maintain and/or improve planarization standards while operating at low downforce. In this study, electrochemical mechanical planarization (ECMP) is considered as an alternative and/or an extension to current CMP processes. ECMP is unique due to the combination of an applied voltage to oxidize Cu and an abrasion from a polishing pad, which potentially allows the system to achieve high levels of planarization through the use of an appropriately tailored electrolyte. An electrolyte containing 1.0 M potassium phosphate salt concentration with a pH value of 2 and a benzotriazole (BTA) concentration of 0.001 M was tested for its planarization capability on patterned Cu structures using a custom built ECMP tool. Feature sizes of the Cu structures were varied from 1 to 6 μm. Similar planarization results were achieved using three pad types. All experiments were performed at 0.5 V versus Ag/AgCl reference. The average step height reduction (SHR) was ~840 nm while the decrease in the average metal thickness removed (λavg) was on the order of ~430 nm. Because features were approximately 50% of the substrate area, the total average metal thickness removed was approximately half of the SHR for all three pad types.







Similar content being viewed by others
References
Zantye PB, Kumar A, Sikder AK (2004) Mater Sci Eng R Rep 45:89
Ishikawa A, Shishida Y, Yamanishi T, Hata N, Nakayama T et al (2006) J Electrochem Soc 153:G692
Aksu S, Emesh I, Uzoh C, Basol B (2006) ECS Trans 2
Goonetilleke PC, Roy D (2008) Appl Surf Sci 254:2696
West AC, Deligianni H, Andricacos PC (2005) IBM J Res Dev 49:37
Liu FQ, Du TB, Duboust A, Tsai S, Hsu WY (2006) J Electrochem Soc 153:C377
Oh YJ, Park GS, Chung CH (2006) J Electrochem Soc 153:G617
Jeong S, Lee S, Jeong H (2008) Microelectron Eng 85:2236
Suni II, Du B (2005) IEEE Trans Semicond Manuf 18:341
Truque D, Xie X, Boning D (2007) Mater Res Soc Symp Proc 991:315
Shattuck KG, Lin J-Y, Cojocaru P, West AC (2008) Electrochim Acta 53:8211
Muthukumaran A, Lowalekar V, Raghavan S (2006) Mater Res Soc Symp Proc 914:231
West AC, Shao I, Deligianni H (2005) J Electrochem Soc 152:C652
Tripathi A, Burkhard C, Suni II, Li Y, Doniat F et al (2008) J Electrochem Soc 155:H918
Lin J-Y, West AC, Wan C-C (2008) J Electrochem Soc 155:H396
Finsgar M, Lesar A, Kokalj A, Milosev I (2008) Electrochim Acta 53:8287
Willey MJ, West AC (2007) J Electrochem Soc 154:D156
Vidal R, West AC (1995) J Electrochem Soc 142:2689
Vidal R, West AC (1995) J Electrochem Soc 142:2682
Acknowledgments
We would like to kindly thank Jeng-Yu Lin and Neha Solanki for their help in fabricating patterned structures. Additionally, we thank the Semiconductor Research Corporation, under task number 425.016, for financially supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shattuck, K.G., West, A.C. An investigation of phosphate based ECMP electrolyte performance on feature scale planarization. J Appl Electrochem 39, 1719–1724 (2009). https://doi.org/10.1007/s10800-009-9865-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9865-7