Abstract
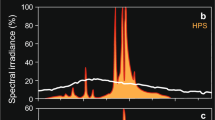
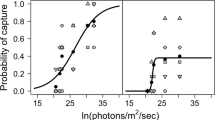
Foraging characteristics of siscowet lake trout (Salvelinus namaycush siscowet) on deepwater sculpin (Myoxocephalus thompsonii) were studied under ecologically relevant downwelling light intensities (9.0 × 108 to 1.62 × 1011 photons m−2 s−1) and emission spectrum (500–510 nm) on varying substrates (gravel, sand, and black fabric). Siscowet reaction distance within our trials increased with light intensity up to 6.0 × 109 photons m−2 s−1, after which reaction distance remained constant with additional increases in light intensity following the Michaelis–Menten saturation function. Reaction distances were not affected by substrate type under any light intensity. The number of prey captures also increased with increasing light intensity, with most orientations toward prey occurring within the siscowet’s forward sector (± 0°–60°, where 0° represents the tip of the siscowet rostrum). Finally, the overall probability of prey capture was positively related to reaction distance at each light intensity. Results suggest that siscowet can visually forage on benthic prey at great depth in Lake Superior, and reaction distance (≤ 27 cm) to sculpin may not diminish until depths exceed 200 m (6.00 × 109 photons m−2 s−1).








Similar content being viewed by others
Change history
11 May 2020
Due to a mistake during the production process, the online/HTML version of the original article was published under the terms of the Creative Commons Attribution 4.0 International License. The article is not open access and no special license applies. The original article has been corrected.
References
Ahrenstorff, T. D., T. R. Hrabik, J. D. Stockwell, D. L. Yule & G. G. Sass, 2011. Seasonally dynamic diel vertical migrations of Mysis diluviana, coregonine fishes, and siscowet lake trout in the pelagia of western Lake Superior. Transactions of the American Fisheries Society 140: 1504–1520.
Aksnes, D. L. & A. C. W. Utne, 1997. A revised model of visual range in fish. Sarsia 82: 137–147.
Beauchamp, D. A., C. M. Baldwin, J. L. Vogel & C. P. Gubala, 1999. Estimating diel, depth-specific foraging opportunities with a visual encounter rate model for pelagic piscivores. Canadian Journal of Fisheries and Aquatic Sciences 56: 128–139.
Beauchamp, D. A., A. D. Cross, J. L. Armstrong, K. W. Myers, J. H. Moss, J. L. Boldt & L. J. Haldorson, 2007. Bioenergetic responses by Pacific salmon to climate and ecosystem variation. North Pacific Anadromous Fish Commission Bulletin 4: 257–269.
Bond, C. E., 1996. Biology of Fishes. Saunders College Publishing, Fort Worth.
Boscarino, B. T., L. G. Rudstam, J. Tirabassi, J. Janssen & E. R. Loew, 2010. Light effects on alewife-mysid interactions in Lake Ontario: a combined sensory physiology, behavioral, and spatial approach. Limnology and Oceanography 55: 2061–2072.
Brandt, S. B. & S. P. Madon, 1986. Rainbow smelt (Osmerus mordax) predation on slimy sculpin (Cottus cognatus) in Lake Ontario. Journal of Great Lakes Research 12: 322–325.
Carmichael, G. J., J. R. Tomasso, B. A. Simco & K. B. Davis, 1984. Characterization and alleviation of stress associated with hauling largemouth bass. Transactions of the American Fisheries Society 113: 778–785.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Cerri, R. D. & D. F. Fraser, 1983. Predation and risk in foraging minnows: balancing conflicting demands. The American Naturalist 121: 552–561.
Crowder, L. B., W. E. Cooper, 1979. Structural complexity and fish–prey interactions in ponds: a point of view. In: Response of Fish to Habitat Structure in Standing Water. American Fisheries Society, North Central Division, Special Publication 6: 2–10.
Crowder, L. B. & W. E. Cooper, 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Crowder, L. B., J. J. Magnuson & S. B. Brandt, 1981. Complementarity in the use of food and thermal habitat by Lake Michigan fishes. Canadian Journal of Fisheries and Aquatic Sciences 38: 662–668.
Cramer, C. E., K. R. Lykke, J. T. Woodward & A. W. Smith, 2013. Precise measurement of lunar spectral irradiance at visible wavelengths. Journal of Research of the National Institute of Standards and Technology 118: 396.
De Robertis, A., C. H. Ryer, A. Veloza & R. D. Brodeur, 2003. Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Canadian Journal of Fisheries and Aquatic Sciences 60: 1517–1526.
Dill, L. M., 1983. Adaptive flexibility in the foraging behavior of fishes. Canadian Journal of Fisheries and Aquatic Sciences 40: 398–408.
Dill, L. M. & A. H. Fraser, 1984. Risk of predation and the feeding behavior of juvenile coho salmon (Oncorhynchus kisutch). Behavioral Ecology and Sociobiology 16: 65–71.
Duke-Elder, S., 1958. A Century of International Ophthalmology, 1857–1957. Kimpton.
Eggers, D. M., 1977. The nature of prey selection by planktivorous fish. Ecology 58: 46–59.
Ellis, T., B. R. Howell & R. N. Hughes, 1997. The cryptic responses of hatchery-reared sole to a natural sand substratum. Journal of Fish Biology 51: 389–401.
Fahnenstiel, G. L., C. L. Schelske & R. A. Moll, 1984. In situ quantum efficiency of Lake Superior phytoplankton. Journal of Great Lakes Research 10: 399–406.
Gorman, O.T., & T. D. Keyler, 2016. A Hyperbaric Holding & Transport Vessel for Collection of Deepwater Fishes for Research and Broodstock Development. U.S. Geological Survey.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012a. Habitat use by fishes of Lake Superior. I. Diel patterns of habitat use in nearshore and offshore waters of the Apostle Islands region. Aquatic Ecosystem Health & Management 15: 333–354.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012b. Habitat use by fishes of Lake Superior. II. Consequences of diel habitat use for habitat linkages and habitat coupling in nearshore and offshore waters. Aquatic Ecosystem Health & Management 15: 355–368.
Hansen, A. G., D. A. Beauchamp & E. R. Schoen, 2013. Visual prey detection responses of piscivorous trout and salmon: effects of light, turbidity, and prey size. Transactions of the American Fisheries Society 142: 854–867.
Harrington, K. A., T. R. Hrabik & A. F. Mensinger, 2015. Visual sensitivity of deepwater fishes in Lake Superior. PLoS ONE 10: e0116173.
Harvey, C. J., S. T. Schram & J. F. Kitchell, 2003. Trophic relationships among lean and siscowet lake trout in Lake Superior. Transactions of the American Fisheries Society 132: 219–228.
Houtman, R. & L. M. Dill, 1994. The influence of substrate color on the alarm response of tidepool sculpins (Oligocottus maculosus; Pisces, Cottidae). Ethology 96: 147–154.
Howick, G. L. & W. J. O’Brien, 1983. Piscivorous feeding behavior of largemouth bass: an experimental analysis. Transactions of the American Fisheries Society 112: 508–516.
Hrabik, T. R., O. P. Jensen, S. J. D. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. I. Changes in vertical migration of coregonids in response to varying predation risk. Canadian Journal of Fisheries and Aquatic Sciences 63: 2286–2295.
Hutchinson, G. E., 1957. A Treatise on Limnology. Geology, Physics, and Chemistry, Vol. 1. Wiley, New York.
Jensen, O. P., T. R. Hrabik, S. J. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. II. Modeling trade-offs at an intermediate trophic level. Canadian Journal of Fisheries and Aquatic Sciences 63: 2296–2307.
Jerome, J. H., R. P. Bukata & J. E. Bruton, 1983. Spectral Attenuation and Irradiance in the Laurentian Great Lakes. Journal of Great Lakes Research 9: 60–68.
Johnsen, S., 2012. The Optics of Life: A Biologist’s Guide to Light. Princeton University Press, New Jersey.
Keyler, T. D., T. R. Hrabik, C. L. Austin, O. T. Gorman & A. F. Mensinger, 2015. Foraging mechanisms of siscowet lake trout (Salvelinus namaycush siscowet) on pelagic prey. Journal of Great Lakes Research 41: 1162–1171.
Kerfoot, W. C. & A. Sih, 1987. Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover.
Land, M. F. & D. E. Nilsson, 2012. Animal Eyes. Oxford University Press, Oxford.
Mazur, M. M. & D. A. Beauchamp, 2003. A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environmental Biology of Fishes 67: 397–405.
Miner, J. G. & R. A. Stein, 1996. Detection of predators and habitat choice by small bluegills: effects of turbidity and alternative prey. Transactions of the American Fisheries Society 125: 97–103.
Mittelbach, G. G., 1981. Foraging efficiency and body size: a study of optimal diet and habitat use by bluegills. Ecology 62: 1370–1386.
Muntz, W. R., 1990. Stimulus, environment and vision in fishes. The visual System of Fish. Springer, Dordrecht.
Munz, F. W. & W. N. McFarland, 1965. A suggested hereditary mechanism for visual pigments of chars (Salvelinus spp.). Nature 206: 955.
Munz, F. W. & W. N. McFarland, 1977. Evolutionary adaptations of fishes to the photic environment. The Visual System in Vertebrates. Springer, Berlin.
O’Neill, R. V., D. L. DeAngelis, J. J. Pastor, B. J. Jackson & W. M. Post, 1989. Multiple nutrient limitations in ecological models. Ecological Modelling 46: 147–163.
Palmer, L. M., M. Deffenbaugh & A. F. Mensinger, 2005. Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). Journal of Experimental Biology 208: 3441–3450.
Pratt, T. C., O. T. Gorman, W. P. Mattes, J. T. Myers, H. R. Quinlan, D. R. Schreiner, M. J. Seider, S. P. Sitar, D. L. Yule, & P. M. Yurista, 2016. The state of Lake Superior in 2011 [online]. Available from: http://www.glfc.org/pubs/SpecialPubs/Sp16_01.pdf [accessed 3 November 2017].
Price, N. N. & A. F. Mensinger, 1999. Predator-prey interactions of juvenile toadfish, Opsanus tau. The Biological Bulletin 197: 246–247.
Prejs, A., 1987. Risk of predation and feeding rate in tropical freshwater fishes: field evidence. Oecologia 72: 259–262.
Richmond, H. E., T. R. Hrabik & A. F. Mensinger, 2004. Light intensity, prey detection and foraging mechanisms of age-0 year yellow perch. Journal of Fish Biology 65: 195–205.
Roth, B. M., K. A. Rose, L. P. Rozas & T. J. Minello, 2008. Relative influence of habitat fragmentation and inundation on brown shrimp Farfantepenaeus aztecus production in northern Gulf of Mexico salt marshes. Marine Ecology Progress Series 359: 185–202.
Ruxton, G. D., T. N. Sherratt & M. P. Speed, 2004. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press, Oxford.
Sass, G. G., C. M. Gille, J. T. Hinke & J. F. Kitchell, 2006. Whole-lake influences of littoral structural complexity and prey body morphology on fish predator–prey interactions. Ecology of Freshwater Fish 15: 301–308.
Scott, W. B. & E. J. Crossman, 1973. Freshwater fishes of Canada. Journal of the Fisheries Research Board of Canada 184: 966.
Selegby, J. H., & M. H. Hoff, 1996. Seasonal Bathymetric Distribution of 16 Fishes in Lake Superior 1958-75 (No. BSR-7). National Biological Service, Ashland WI, Great Lakes Science Center.
Sierszen, M. E., T. R. Hrabik, J. D. Stockwell, A. M. Cotter, J. C. Hoffman & D. L. Yule, 2014. Depth gradients in food-web processes linking habitats in large lakes: Lake Superior as an exemplar ecosystem. Freshwater Biology 59: 2122–2136.
Sih, A., 1986. Antipredator responses and the perception of danger by mosquito larvae. Ecology 67: 434–441.
Sitar, S. P., H. M. Morales, M. T. Mata, B. B. Bastar, D. M. Dupras, G. D. Kleaver & K. D. Rathbun, 2008. Survey of Siscowet Lake Trout at Their Maximum Depth in Lake Superior. Journal of Great Lakes Research 34: 276–286.
Sowersby, W., R. M. Thompson & B. B. M. Wong, 2015. Invasive predator influences habitat preferences in a freshwater fish. Environmental Biology of Fishes 99: 187–193.
Stevens, M. & I. C. Cuthill, 2006. Disruptive coloration, crypsis and edge detection in early visual processing. Proceedings of the Royal Society B 273: 2141–2147.
Stockwell, J. D., T. R. Hrabik, O. P. Jensen, D. L. Yule & M. Balge, 2010. Empirical evaluation of predator-driven diel vertical migration in Lake Superior. Canadian Journal of Fisheries and Aquatic Sciences 67: 473–485.
van der Meer, H. J. & G. C. Anker, 1984. Retinal resolving power and sensitivity of the photopic system in seven haplochromine species (Cichlidae, Teleostei). Netherlands Journal of Zoology 34: 197–209.
Virostek, K., & B. Franckowiak, 2014. What can you see in the dark? The effects of contrast, light, and age on contrast sensitivity in low light. Journal of Emerging Investigators 1–4.
Vogel, J. L. & D. A. Beauchamp, 1999. Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Canadian Journal of Fisheries and Aquatic Sciences 56: 1293–1297.
Ware, D. M., 1973. Risk of epibenthic prey to predation by rainbow trout (Salmo gairdneri). Journal of the Fisheries Research Board of Canada 30: 787–797.
Wentworth, C. K., 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology 30 (5):377–392.
Werner, E. E. & D. J. Hall, 1974. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55: 1042–1052.
Werner, E. E. & D. J. Hall, 1979. Foraging efficiency and habitat switching in competing sunfishes. Ecology 60: 256–264.
Acknowledgements
For assistance with collection of deepwater fishes, the authors thank the crew of the RV Kiyi. Funding for this project was provided by the US Geological Survey, Lake Superior Biological Station, University of Minnesota Duluth Biology Department, and National Science Foundation Grants IOS 1354745 and DOB 1359230 (AFM). The authors are grateful for the help and support from I. Harding, M. Pawlowski, J. Dobosenski, Minnesota Sea Grant, and the McNair Scholars Program at the University of Wisconsin-Superior. A special thanks to M. Joyce for help with statistical aspects of the paper, B. Matthias for his helpful editorial comments, and R. Mahling for his modeling expertise. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: C. E. Adams, C. R. Bronte, M. J. Hansen, R. Knudsen, & M. Power/Charr Biology, Ecology and Management
Rights and permissions
About this article
Cite this article
Keyler, T.D., Hrabik, T.R., Mensinger, A.F. et al. Effect of light intensity and substrate type on siscowet lake trout (Salvelinus namaycush siscowet) predation on deepwater sculpin (Myoxocephalus thompsonii). Hydrobiologia 840, 77–88 (2019). https://doi.org/10.1007/s10750-019-3944-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3944-5