Abstract
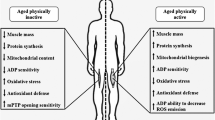
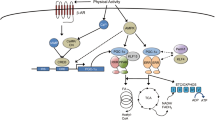
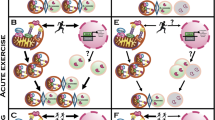
Changes in mitochondrial capacity and quality play a critical role in skeletal and cardiac muscle dysfunction. In vivo measurements of mitochondrial capacity provide a clear link between physical activity and mitochondrial function in aging and heart failure, although the cause and effect relationship remains unclear. Age-related decline in mitochondrial quality leads to mitochondrial defects that affect redox, calcium, and energy-sensitive signaling by altering the cellular environment that can result in skeletal muscle dysfunction independent of reduced mitochondrial capacity. This reduced mitochondrial quality with age is also likely to sensitize skeletal muscle mitochondria to elevated angiotensin or beta-adrenergic signaling associated with heart failure. This synergy between aging and heart failure could further disrupt cell energy and redox homeostasis and contribute to exercise intolerance in this patient population. Therefore, the interaction between aging and heart failure, particularly with respect to mitochondrial dysfunction, should be a consideration when developing strategies to improve quality of life in heart failure patients. Given the central role of the mitochondria in skeletal and cardiac muscle dysfunction, mitochondrial quality may provide a common link for targeted interventions in these populations.
Similar content being viewed by others
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics C, Stroke Statistics S (2010) Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121(7):948–954
Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA (2001) Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56(5):B209–B217
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 45(7–8):466–472
Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37(6):755–767
Fisher-Wellman KH, Neufer PD (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 23(3):142–153
Jiang P, Du W, Mancuso A, Wellen KE, Yang X (2013) Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493(7434):689–693
Merritt TJ, Kuczynski C, Sezgin E, Zhu CT, Kumagai S, Eanes WF (2009) Quantifying interactions within the NADP(H) enzyme network in Drosophila melanogaster. Genetics 182(2):565–574
Gameiro PA, Laviolette LA, Kelleher JK, Iliopoulos O, Stephanopoulos G (2013) Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem 288(18):12967–12977
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD (2009) Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54(20):1891–1898
Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS (2014) Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3:6
Feissner RF, Skalska J, Gaum WE, Sheu SS (2009) Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14:1197–1218
Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM (2002) Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med 33(6):755–764
Kramer PA, Duan J, Qian WJ, Marcinek DJ (2015) The measurement of reversible redox dependent post-translational modifications and their regulation of mitochondrial and skeletal muscle function. Front Physiol 6:347
Fitts RH (1994) Cellular mechanisms of muscle fatigue. Physiol Rev 74(1):49–94
Okamoto K, Wang W, Rounds J, Chambers EA, Jacobs DO (2001) ATP from glycolysis is required for normal sodium homeostasis in resting fast-twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab 281(3):E479–E488
Homsher E, Kean CJ (1978) Skeletal muscle energetics and metabolism. Annu Rev Physiol 40:93–131
Rall JA (1985) Energetic aspects of skeletal muscle contraction: implications of fiber types. Exerc Sport Sci Rev 13:33–74
Blei ML, Conley KE, Kushmerick MJ (1993) Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 465:203–222
Conley KE, Jubrias SA, Esselman PC (2000) Oxidative capacity and ageing in human muscle. J Physiol 526(Pt 1):203–210
Siegel MP, Kruse SE, Knowels G, Salmon A, Beyer R, Xie H, Van Remmen H, Smith SR, Marcinek DJ (2011) Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS One 6(11):e26963
Siegel MP, Wilbur T, Mathis M, Shankland EG, Trieu A, Harper ME, Marcinek DJ (2012) Impaired adaptability of in vivo mitochondrial energetics to acute oxidative insult in aged skeletal muscle. Mech Ageing Dev 133(9–10):620–628
Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE (2005) Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol 569(Pt 2):467–473
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE (2002) Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346(11):793–801
Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG (2005) Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 112(5):674–682
Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW (2015) Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 12(3):294–304
Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, Haykowsky MJ, Brubaker PH, Kitzman DW (2016) Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail 4(8):636–645
Hoppeler H (1990) The different relationship of VO2max to muscle mitochondria in humans and quadrupedal animals. Respir Physiol 80(2–3):137–145
Heath GW, Hagberg JM, Ehsani AA, Holloszy JO (1981) A physiological comparison of young and older endurance athletes. J Appl Physiol Respir Environ Exerc Physiol 51(3):634–640
Rogers MA, Hagberg JM, Martin WH 3rd, Ehsani AA, Holloszy JO (1990) Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol (1985) 68(5):2195–2199
Zizola C, Schulze PC (2013) Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev 18(5):623–630
Mancini DM, Ferraro N, Tuchler M, Chance B, Wilson JR (1988) Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol 62(17):1234–1240
Dudley GA, Tullson PC, Terjung RL (1987) Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem 262(19):9109–9114
Cadenas E (2004) Mitochondrial free radical production and cell signaling. Mol Asp Med 25(1–2):17–26
Garcia J, Han D, Sancheti H, Yap LP, Kaplowitz N, Cadenas E (2010) Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem 285(51):39646–39654
Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T (2013) The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15(12):1464–1472
Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R (1999) Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A 96(24):13807–13812
Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G (2000) Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem 275(20):15305–15313
Kemp GJ, Ahmad RE, Nicolay K, Prompers JJ (2015) Quantification of skeletal muscle mitochondrial function by 31P magnetic resonance spectroscopy techniques: a quantitative review. Acta Physiol (Oxf) 213(1):107–144
Campbell MD, Marcinek DJ (2016) Evaluation of in vivo mitochondrial bioenergetics in skeletal muscle using NMR and optical methods. Biochim Biophys Acta 1862(4):716–724
Lanza IR, Bhagra S, Nair KS, Port JD (2011) Measurement of human skeletal muscle oxidative capacity by 31P-MR spectroscopy: a cross-validation with in vitro measurements. J Magn Reson Imaging 34(5):1143–1150
Percival JM, Siegel MP, Knowels G, Marcinek DJ (2013) Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of Duchenne muscular dystrophy are not alleviated by PDE5 inhibition. Hum Mol Genet 22(1):153–167
Broskey NT, Boss A, Fares EJ, Greggio C, Gremion G, Schluter L, Hans D, Kreis R, Boesch C, Amati F (2015) Exercise efficiency relates with mitochondrial content and function in older adults. Physiol Rep 3(6)
Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE, Goodpaster BH (2013) Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 68(4):447–455
Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK (2012) Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113(2):175–183
Ryan TE, Southern WM, Brizendine JT, McCully KK (2013) Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc 45(12):2346–2352
Ryan TE, Southern WM, Reynolds MA, McCully KK (2013) A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 115(12):1757–1766
De Blasi RA, Almenrader N, Aurisicchio P, Ferrari M (1997) Comparison of two methods of measuring forearm oxygen consumption (VO2) by near infrared spectroscopy. J Biomed Opt 2(2):171–175
Van Beekvelt MCP, Colier WNJM, Wevers RA, Van Engelen BGM (2001) Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle, vol 90. vol 2
Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA (2012) Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37(1):88–99
Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE (2007) Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A 104(3):1057–1062
Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ (2013) Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 12(5):763–771
Southern WM, Ryan TE, Kepple K, Murrow JR, Nilsson KR, McCully KK (2015) Reduced skeletal muscle oxidative capacity and impaired training adaptations in heart failure. Physiol Rep 3(4)
Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD (2011) Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 13(12):1296–1304
Mancini DM, Henson D, LaManca J, Levine S (1994) Evidence of reduced respiratory muscle endurance in patients with heart failure. J Am Coll Cardiol 24(4):972–981
Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR (1986) Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation 73(6):1127–1136
Mancini DM, Coyle E, Coggan A, Beltz J, Ferraro N, Montain S, Wilson JR (1989) Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation 80(5):1338–1346
Hart CR, Layec G, Trinity JD, Liu X, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS (2015) Evidence of preserved oxidative capacity and oxygen delivery in the plantar flexor muscles with age. J Gerontol A Biol Sci Med Sci 70(9):1067–1076
Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS (2015) Impact of age on exercise-induced ATP supply during supramaximal plantar flexion in humans. Am J Physiol Regul Integr Comp Physiol 309(4):R378–R388
Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA (2003) Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol (1985) 95(6):2361–2369
Conley KE, Amara CE, Bajpeyi S, Costford SR, Murray K, Jubrias SA, Arakaki L, Marcinek DJ, Smith SR (2013) Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary versus active subjects. J Clin Endocrinol Metab 98(1):129–136
Santanasto AJ, Glynn NW, Jubrias SA, Conley KE, Boudreau RM, Amati F, Mackey DC, Simonsick EM, Strotmeyer ES, Coen PM, Goodpaster BH, Newman AB (2015) Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol A Biol Sci Med Sci 70(11):1379–1385
Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE (2008) Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods 46(4):312–318
Nyberg M, Mortensen SP, Cabo H, Gomez-Cabrera MC, Vina J, Hellsten Y (2014) Roles of sedentary aging and lifelong physical activity in exchange of glutathione across exercising human skeletal muscle. Free Radic Biol Med 73:166–173
Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F, Jansen-Durr P (2007) Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 6(2):245–256
Jackson MJ (2013) Interactions between reactive oxygen species generated by contractile activity and aging in skeletal muscle? Antioxid Redox Signal 19(8):804–812
Jackson MJ (2013) Monitoring of hydrogen peroxide and other reactive oxygen and nitrogen species generated by skeletal muscle. Methods Enzymol 528:279–300
Marcinek DJ, Siegel MP (2013) Targeting redox biology to reverse mitochondrial dysfunction. Aging (Albany NY) 5(8):588–589
Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R (2013) Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18(2):239–250
O'Rourke B, Van Eyk JE, Foster DB (2011) Mitochondrial protein phosphorylation as a regulatory modality: implications for mitochondrial dysfunction in heart failure. Congest Heart Fail 17(6):269–282
Kelly DP, Scarpulla RC (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18(4):357–368
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106(7):847–856
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127(2):397–408
Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS (2008) Endurance exercise as a countermeasure for aging. Diabetes 57(11):2933–2942
Knutti D, Kralli A (2001) PGC-1, a versatile coactivator. Trends Endocrinol Metab 12(8):360–365
Johnson ML, Robinson MM, Nair KS (2013) Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab 24(5):247–256
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A 106(48):20405–20410
Ji LL, Kang C (2015) Role of PGC-1alpha in sarcopenia: etiology and potential intervention—a mini-review. Gerontology 61(2):139–148
Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA (2008) Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7(1):2–12
Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP, Garduno-Garcia Jde J, Abdul-Ghani M, Richardson A, DeFronzo RA, Mandarino L, Van Remmen H, Musi N (2011) Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60(8):2051–2060
Toth MJ, Miller MS, Ward KA, Ades PA (2012) Skeletal muscle mitochondrial density, gene expression, and enzyme activities in human heart failure: minimal effects of the disease and resistance training. J Appl Physiol (1985) 112(11):1864–1874
Middlekauff HR, Verity MA, Horwich TB, Fonarow GC, Hamilton MA, Shieh P (2013) Intact skeletal muscle mitochondrial enzyme activity but diminished exercise capacity in advanced heart failure patients on optimal medical and device therapy. Clin Res Cardiol 102(8):547–554
Zizola C, Kennel PJ, Akashi H, Ji R, Castillero E, George I, Homma S, Schulze PC (2015) Activation of PPARdelta signaling improves skeletal muscle oxidative metabolism and endurance function in an animal model of ischemic left ventricular dysfunction. Am J Physiol Heart Circ Physiol 308(9):H1078–H1085
Schrepper A, Schwarzer M, Schope M, Amorim PA, Doenst T (2012) Biphasic response of skeletal muscle mitochondria to chronic cardiac pressure overload—role of respiratory chain complex activity. J Mol Cell Cardiol 52(1):125–135
Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS (2011) Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58(1):73–82
Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M (2014) Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306(9):H1364–H1370
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884S–8890
Houtkooper RH, Canto C, Wanders RJ, Auwerx J (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 31(2):194–223
Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA (2013) Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155(7):1624–1638
Kruse SE, Karunadharma PP, Basisty N, Johnson R, Beyer RP, MacCoss MJ, Rabinovitch PS, Marcinek DJ (2016) Age modifies respiratory complex I and protein homeostasis in a muscle type-specific manner. Aging Cell 15(1):89–99
Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q (2004) In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol 287(4):H1813–H1820
Marin-Garcia J, Goldenthal MJ, Moe GW (2001) Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res 52(1):103–110
Rosca MG, Hoppel CL (2010) Mitochondria in heart failure. Cardiovasc Res 88(1):40–50
Hoppel CL, Tandler B, Fujioka H, Riva A (2009) Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol 41(10):1949–1956
Laitano O, Ahn B, Patel N, Coblentz PD, Smuder AJ, Yoo JK, Christou DD, Adhihetty PJ, Ferreira LF (2016) Pharmacological targeting of mitochondrial reactive oxygen species counteracts diaphragm weakness in chronic heart failure. J Appl Physiol (1985) 120(7):733–742
Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD (2003) A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J 22(16):4103–4110
Mailloux RJ, Adjeitey CN, Xuan JY, Harper ME (2012) Crucial yet divergent roles of mitochondrial redox state in skeletal muscle vs. brown adipose tissue energetics. FASEB J 26(1):363–375
Jackson MJ (2009) Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic Biol Med 47(9):1267–1275
Jackson MJ (2008) Redox regulation of skeletal muscle. IUBMB Life 60(8):497–501
Forman HJ, Ursini F, Maiorino M (2014) An overview of mechanisms of redox signaling. J Mol Cell Cardiol 73:2–9
Rosca MG, Hoppel CL (2013) Mitochondrial dysfunction in heart failure. Heart Fail Rev 18(5):607–622
Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 148(7):3441–3448
Benson DW, Foley-Nelson T, Chance WT, Zhang FS, James JH, Fischer JE (1991) Decreased myofibrillar protein breakdown following treatment with clenbuterol. J Surg Res 50(1):1–5
Rosca MG, Tandler B, Hoppel CL (2013) Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol 55:31–41
Rosca MG, Hoppel CL (2009) New aspects of impaired mitochondrial function in heart failure. J Bioenerg Biomembr 41(2):107–112
DiPilato LM, Cheng X, Zhang J (2004) Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A 101(47):16513–16518
Rosca M, Minkler P, Hoppel CL (2011) Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta 1807(11):1373–1382
Brink M, Wellen J, Delafontaine P (1996) Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest 97(11):2509–2516
Wang Y, Seto SW, Golledge J (2014) Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail Rev 19(2):187–198
Kadoguchi T, Kinugawa S, Takada S, Fukushima A, Furihata T, Homma T, Masaki Y, Mizushima W, Nishikawa M, Takahashi M, Yokota T, Matsushima S, Okita K, Tsutsui H (2015) Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp Physiol 100(3):312–322
Takada S, Kinugawa S, Hirabayashi K, Suga T, Yokota T, Takahashi M, Fukushima A, Homma T, Ono T, Sobirin MA, Masaki Y, Mizushima W, Kadoguchi T, Okita K, Tsutsui H (2013) Angiotensin II receptor blocker improves the lowered exercise capacity and impaired mitochondrial function of the skeletal muscle in type 2 diabetic mice. J Appl Physiol (1985) 114(7):844–857
Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS (2011) Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108(7):837–846
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW (1994) Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74(6):1141–1148
Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T (2002) Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90(4):E58–E65
Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P (2011) Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409(2):217–221
Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS (2006) Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281(46):35137–35146
Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS (2014) Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13(3):529–539
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14(2):196–207
Rullman E, Andersson DC, Melin M, Reiken S, Mancini DM, Marks AR, Lund LH, Gustafsson T (2013) Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J Heart Lung Transplant 32(9):925–929
Umanskaya A, Santulli G, Xie W, Andersson DC, Reiken SR, Marks AR (2014) Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc Natl Acad Sci U S A 111(42):15250–15255
Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining "clean" cells. Autophagy 1(3):131–140
Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT (2003) Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol 38(8):863–876
Coleman R, Silbermann M, Gershon D, Reznick AZ (1987) Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology 33(1):34–39
Brunk UT, Terman A (2002) The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269(8):1996–2002
Dayan D, Abrahami I, Buchner A, Gorsky M, Chimovitz N (1988) Lipid pigment (lipofuscin) in human perioral muscles with aging. Exp Gerontol 23(2):97–102
Dayan D, David R, Buchner A (1979) Lipofuscin in human tongue muscle. J Oral Pathol 8(2):121–125
Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS (2009) Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119(21):2789–2797
Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS (2010) Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9(4):536–544
Zalk R, Lehnart SE, Marks AR (2007) Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76:367–385
Acknowledgments
This publication was supported by awards from the National Institute on Aging of the National Institutes of Health AG001751 and AG000057 and a Breakthrough in Gerontology Award from the American Federation for Aging Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Marcinek serves as a paid consultant to Stealth BioTherapeutics. Stealth is developing the compound elamipretide (SS-31) for clinical use. Sophia Liu has no conflict of interest to report.
Rights and permissions
About this article
Cite this article
Liu, S.Z., Marcinek, D.J. Skeletal muscle bioenergetics in aging and heart failure. Heart Fail Rev 22, 167–178 (2017). https://doi.org/10.1007/s10741-016-9586-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9586-z