Abstract
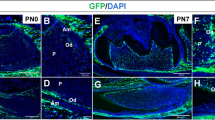
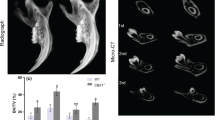
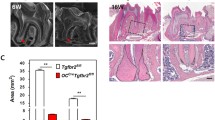
Bone morphogenetic protein 1 (BMP1) and tolloid-like 1 (TLL1) belong to the BMP1/tolloid-like proteinase family, which cleaves secretory proteins. The constitutive deletion of the Bmp1 or Tll1 genes causes perinatal or embryonic lethality in mice. In this study, we first studied the β-galactosidase activity in mice in which an IRES-lacZ-Neo cassette was inserted in the intron of either the Bmp1 or the Tll1 gene; the β-galactosidase activities were used to reflect the expression of endogenous Bmp1 and Tll1, respectively. Our X-gal staining results showed that the odontoblasts in the tooth and cells in the periodontal ligament express both Bmp1 and Tll1. We then created Bmp1 flox/flox and Tll1 flox/flox mice by removing the IRES-lacZ-Neo cassette. By breeding 2.3 kb Col1a1-Cre mice with the Bmp1 flox/flox and Tll1 flox/flox mice, we further generated Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice in which both Bmp1 and Tll1 were inactivated in the Type I collagen-expressing cells. We employed X-ray radiography, histology and immunohistochemistry approaches to characterize the Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice. Our results showed that the molars of the Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice had wider predentin, thinner dentin and larger pulp chambers than those of the normal controls. The dentinal tubules of the molars in the Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice appeared disorganized. The level of dentin sialophosphoprotein in the molars of the 6-week-old Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice was lower than in the normal controls. The periodontal ligaments of the Col1a1-Cre;Bmp1 flox/flox;Tll1 flox/flox mice were disorganized and had less fibrillin-1. Our findings indicate that the proteinases encoded by Bmp1 and Tll1 genes play essential roles in the development and maintenance of mouse dentin and periodontal ligaments.












Similar content being viewed by others
References
Butler WT, Brunn JC, Qin C, McKee MD (2002) Extracellular matrix proteins and the dynamics of dentin formation. Connect Tissue Res 43:301–307
Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, Hogan BL, Greenspan DS (1999) The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development 126:2631–2642
Hopkins DR, Keles S, Greenspan DS (2007) The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 26:508–523
Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS (1996) Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 271:360–362
Lee SJ (2008) Genetic analysis of the role of proteolysis in the activation of latent myostatin. Plos One 3:e1628
Leighton M, Kadler KE (2003) Paired basic/furin-like proprotein convertase cleavage of pro-BMP-1 in the trans-Golgi network. J Biol Chem 278:18478–18484
Li R, Zhang Q (2015) HtrA1 may regulate the osteogenic differentiation of human periodontal ligament cells by TGF-β1. J Mol Histol 46(2):137–144
Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ (1996) The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci USA 93:5127–5130
Liu P, Zhang H, Liu C, Wang X, Chen L, Qin C (2014) Inactivation of Fam20C in cells expressing type I collagen causes periodontal disease in mice. PLoS One 9:e114396
Lönnqvist L, Reinhardt D, Sakai L, Peltonen L (1998) Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet 13:2039–2044
Milewicz DM, Pyeritz RE, Crawford ES, Byers PH (1992) Marfan syndrome: defective synthesis, secretion and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest 89:79–86
Milewicz DM, Grossfield J, Cao S-N, Kielty C, Covitz W, Jewett T (1995) A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest 95:2373–2378
Muir A, Greenspan DS (2011) Metalloproteinases in Drosophila to humans that are central players in developmental processes. J Biol Chem 286:21911–41905
Muir AM, Ren Y, Butz DH, Davis NA, Blank RD, Birk DE, Lee SJ, Rowe D, Feng JQ, Greenspan DS (2014) Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice. Hum Mol Genet 23:3085–3101
Muromachi K, Kamio N, Matsuki-Fukushima M, Nishimura H, Tani-Ishii N, Sugiya H, Matsushima K (2015) CCN2/CTGF expression via cellular uptake of BMP-1 is associated with reparative dentinogenesis. Oral Dis 21:778–784
Qin C, Baba O, Butler WT (2004) Posttranslational modifications of SIBLING proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 15:126–136
Raghunath M, Kielty CM, Steinmann B (1995) Truncated profibrillin of a Marfan patient is of apparent similar size as fibrillin: intracellular retention leads to over-N-glycosylation. J Mol Biol 248:901–909
Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschödrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM (1999) Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci 112:1093–1100
Reynolds SD, Zhang D, Puzas JE, O’Keefe RJ, Rosier RN, Reynolds PR (2000) Cloning of the chick BMP1/Tolloid cDNA and expression in skeletal tissues. Gene 248:233–243
Rios H, Koushik SV, Wang H, Wang J, Zhou HM et al (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25:11131–11144
Ritchie HH, Yee CT, Tang XN, Dong Z, Fuller RS (2012) DSP-PP precursor protein cleavage by tolloid-related-1 protein and by bone morphogenetic protein-1. PLoS One 7:e41110
Romanos GE, Asnani KP, Hingorani D, Deshmukh VL (2014) Periostin: role in formation and maintenance of dental tissues. J Cell Physiol 229:1–5
Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS (1999) Mammalian BMP-1/tolloid-related metalloproteinases, including novel family member mammalian tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 213:283–300
Shiga M, Saito M, Hattori M, Torii C, Kosaki K et al (2008) Characteristic phenotype of immortalized periodontal cells isolated from a Marfan syndrome type I patient. Cell Tissue Res 331:461–472
Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS (2004) Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem 279:980–986
Sterchi EE, Stöcker W, Bond JS (2008) Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med 29:309–328
Suda N, Shiga M, Ganburged G, Moriyama K (2009) Marfan syndrome and its disorder in periodontal tissues. J Exp Zool B Mol Dev Evol 312B:503–509
Sun Y, Lu Y, Chen S, Prasad M, Wang X, Zhu Q, Zhang J, Ball H, Feng J, Butler WT, Qin C (2010) Key proteolytic cleavage site and full-length form of DSPP. J Dent Res 89:498–503
Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL (1996) Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development 122:3587–3595
Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, Hermanns-Lê T, Coucke PJ, De Paepe A, Malfait F (2015) Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J Bone Miner Res 30:1445–1456
Takahara K, Lyons GE, Greenspan DS (1994) Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem 269:32572–32578
Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y (2011) Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp). J Bone Miner Res 2011:220–228
Von Marschall Z, Fisher LW (2010) Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1). Matrix Biol 29:295–303
Yang G, Jiang B, Cai W, Liu S, Zhao S (2016) Hyaluronan and hyaluronan synthases expression and localization in embryonic mouse molars. J Mol Histol 47(4):413–420
Ye X, Zhang J, Yang P (2016) Hyperlipidemia induced by high-fat diet enhances dentin formation and delays dentin mineralization in mouse incisor. J Mol Histol 47(5):467–474
Zhou Z, Yin Y, Jiang F, Niu Y, Wan S, Chen N, Shen M (2016) CBX7 deficiency plays a positive role in dentin and alveolar bone development. J Mol Histol 47(4):401–411
Zhu Q, Prasad M, Kong H, Lu Y, Sun Y, Wang X, Yamoah A, Feng JQ, Qin C (2012a) Partial blocking of mouse DSPP processing by substitution of Gly451-Asp452 bond suggests the presence of secondary cleavage site(s). Connect Tissue Res 53:307–312
Zhu Q, Gibson MP, Liu Q, Liu Y, Lu Y, Wang X, Feng JQ, Qin C (2012b) Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis. J Biol Chem 287:30426–30435
Acknowledgements
This work was supported by the United States National Institutes of Health Grants DE022549 and DE023365. We thank Jeanne Santa Cruz for her assistance with the editing of this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, H., Jani, P., Liang, T. et al. Inactivation of bone morphogenetic protein 1 (Bmp1) and tolloid-like 1 (Tll1) in cells expressing type I collagen leads to dental and periodontal defects in mice. J Mol Hist 48, 83–98 (2017). https://doi.org/10.1007/s10735-016-9708-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-016-9708-x