Abstract
The bisecting GlcNAc is transferred to the core mannose residue of complex or hybrid N-glycans on glycoproteins by the β1,4-N-acetylglucosaminyltransferase III (GlcNAcT-III) or MGAT3. The addition of the bisecting GlcNAc confers unique lectin recognition properties to N-glycans. Thus, LEC10 gain-of-function Chinese hamster ovary (CHO) cells selected for the acquisition of ricin resistance, carry N-glycans with a bisecting GlcNAc, which enhances the binding of the erythroagglutinin E-PHA, but reduces the binding of ricin and galectins-1, -3 and -8. The altered interaction with galactose-binding lectins suggests that the bisecting GlcNAc affects N-glycan conformation. LEC10 mutants expressing polyoma middle T antigen (PyMT) exhibit reduced growth factor signaling. Furthermore, PyMT-induced mammary tumors lacking MGAT3, progress more rapidly than tumors with the bisecting GlcNAc on N-glycans of cell surface glycoproteins. In recent years, evidence for a new paradigm of cell growth control has emerged involving regulation of cell surface residency of growth factor and cytokine receptors via interactions and cross-linking of their branched N-glycans with a lattice of galectin(s). Specific cross-linking of glycoprotein receptors in the lattice regulates their endocytosis, leading to effects on growth factor-induced signaling. This review will describe evidence that the bisecting GlcNAc of N-glycans regulates cellular signaling and tumor progression, apparently through modulating N-glycan/galectin interactions.



Similar content being viewed by others
References
Culyba, E.K., Price, J.L., Hanson, S.R., Dhar, A., Wong, C.H., Gruebele, M., Powers, E.T., Kelly, J.W.: Protein native-state stabilization by placing aromatic side chains in N-glycosylated reverse turns. Science 331(6017), 571–575 (2011). doi:10.1126/science.1198461
Price, J.L., Powers, D.L., Powers, E.T., Kelly, J.W.: Glycosylation of the enhanced aromatic sequon is similarly stabilizing in three distinct reverse turn contexts. Proc Natl Acad Sci U S A 108(34), 14127–14132 (2011). doi:10.1073/pnas.1105880108
Gupta, R., Birch, H., Rapacki, K., Brunak, S., Hansen, J.E.: O-GLYCBASE version 4.0: a revised database of O-glycosylated proteins. Nucleic Acids Res 27(1), 370–372 (1999)
Nishikawa, I., Nakajima, Y., Ito, M., Fukuchi, S., Homma, K., Nishikawa, K.: Computational prediction of o-linked glycosylation sites that preferentially map on intrinsically disordered regions of extracellular proteins. Int J Mol Sci 11(12), 4991–5008 (2010). doi:10.3390/ijms11124991
Gerken, T.A., Jamison, O., Perrine, C.L., Collette, J.C., Moinova, H., Ravi, L., Markowitz, S.D., Shen, W., Patel, H., Tabak, L.A.: Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J Biol Chem 286(16), 14493–14507 (2011). doi:10.1074/jbc.M111.218701
North, S.J., Hitchen, P.G., Haslam, S.M., Dell, A.: Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr Opin Struct Biol 19(5), 498–506 (2009). doi:10.1016/j.sbi.2009.05.005
Zielinska, D.F., Gnad, F., Wisniewski, J.R., Mann, M.: Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141(5), 897–907 (2010). doi:10.1016/j.cell.2010.04.012
Narasimhan, S.: Control of glycoprotein synthesis. UDP-GlcNAc:glycopeptide beta 4-N-acetylglucosaminyltransferase III, an enzyme in hen oviduct which adds GlcNAc in beta 1–4 linkage to the beta-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J Biol Chem 257(17), 10235–10242 (1982)
Brockhausen, I., Narasimhan, S., Schachter, H.: The biosynthesis of highly branched N-glycans: studies on the sequential pathway and functional role of N-acetylglucosaminyltransferases I, II, III, IV, V and VI. Biochimie 70(11), 1521–1533 (1988)
Longmore, G.D., Schachter, H.: Product-identification and substrate-specificity studies of the GDP-L-fucose:2-acetamido-2-deoxy-beta-D-glucoside (FUC goes to Asn-linked GlcNAc) 6-alpha-L-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr Res 100, 365–392 (1982)
Stanley, P., Sundaram, S., Tang, J., Shi, S.: Molecular analysis of three gain-of-function CHO mutants that add the bisecting GlcNAc to N-glycans. Glycobiology 15(1), 43–53 (2005). doi:10.1093/glycob/cwh136
North, S.J., Huang, H.H., Sundaram, S., Jang-Lee, J., Etienne, A.T., Trollope, A., Chalabi, S., Dell, A., Stanley, P., Haslam, S.M.: Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J Biol Chem 285(8), 5759–5775 (2010). doi:10.1074/jbc.M109.068353
Bhattacharyya, R., Bhaumik, M., Raju, T.S., Stanley, P.: Truncated, inactive N-acetylglucosaminyltransferase III (GlcNAc-TIII) induces neurological and other traits absent in mice that lack GlcNAc-TIII. J Biol Chem 277(29), 26300–26309 (2002). doi:10.1074/jbc.M202276200
Song, Y., Aglipay, J.A., Bernstein, J.D., Goswami, S., Stanley, P.: The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res 70(8), 3361–3371 (2010). doi:10.1158/0008-5472.CAN-09-2719
Campbell, C., Stanley, P.: A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J Biol Chem 259(21), 13370–13378 (1984)
Stanley, P., Caillibot, V., Siminovitch, L.: Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell 6(2), 121–128 (1975)
Brisson, J.R., Carver, J.P.: Solution conformation of asparagine-linked oligosaccharides: alpha(1–2)-, alpha(1–3)-, beta(1–2)-, and beta(1–4)-linked units. Biochemistry 22(15), 3671–3680 (1983)
Andre, S., Unverzagt, C., Kojima, S., Frank, M., Seifert, J., Fink, C., Kayser, K., von der Lieth, C.W., Gabius, H.J.: Determination of modulation of ligand properties of synthetic complex-type biantennary N-glycans by introduction of bisecting GlcNAc in silico, in vitro and in vivo. Eur J Biochem/FEBS 271(1), 118–134 (2004)
Takahashi, M., Kuroki, Y., Ohtsubo, K., Taniguchi, N.: Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: their functions and target proteins. Carbohydr Res 344(12), 1387–1390 (2009). doi:10.1016/j.carres.2009.04.031
Stanley, P.: Biological consequences of overexpressing or eliminating N-acetylglucosaminyltransferase-TIII in the mouse. Biochim Biophys Acta 1573(3), 363–368 (2002)
Bhaumik, M., Seldin, M.F., Stanley, P.: Cloning and chromosomal mapping of the mouse Mgat3 gene encoding N-acetylglucosaminyltransferase III. Gene 164(2), 295–300 (1995)
Priatel, J.J., Sarkar, M., Schachter, H., Marth, J.D.: Isolation, characterization and inactivation of the mouse Mgat3 gene: the bisecting N-acetylglucosamine in asparagine-linked oligosaccharides appears dispensable for viability and reproduction. Glycobiology 7(1), 45–56 (1997)
Vagin, O., Tokhtaeva, E., Yakubov, I., Shevchenko, E., Sachs, G.: Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na, K-ATPase beta1 subunit. J Biol Chem 283(4), 2192–2202 (2008). doi:10.1074/jbc.M704713200
Bhaumik, M., Harris, T., Sundaram, S., Johnson, L., Guttenplan, J., Rogler, C., Stanley, P.: Progression of hepatic neoplasms is severely retarded in mice lacking the bisecting N-acetylglucosamine on N-glycans: evidence for a glycoprotein factor that facilitates hepatic tumor progression. Cancer Res 58(13), 2881–2887 (1998)
Isaji, T., Kariya, Y., Xu, Q., Fukuda, T., Taniguchi, N., Gu, J.: Functional roles of the bisecting GlcNAc in integrin-mediated cell adhesion. Methods Enzymol 480, 445–459 (2010). doi:10.1016/S0076-6879(10)80019-9
Hirabayashi, J., Kasai, K.: The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3(4), 297–304 (1993)
Houzelstein, D., Goncalves, I.R., Fadden, A.J., Sidhu, S.S., Cooper, D.N., Drickamer, K., Leffler, H., Poirier, F.: Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol 21(7), 1177–1187 (2004). doi:10.1093/molbev/msh082
Dagher, S.F., Wang, J.L., Patterson, R.J.: Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A 92(4), 1213–1217 (1995)
Di Lella, S., Sundblad, V., Cerliani, J.P., Guardia, C.M., Estrin, D.A., Vasta, G.R., Rabinovich, G.A.: When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50(37), 7842–7857 (2011). doi:10.1021/bi201121m
Seelenmeyer, C., Wegehingel, S., Tews, I., Kunzler, M., Aebi, M., Nickel, W.: Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1. J Cell biol 171(2), 373–381 (2005). doi:10.1083/jcb.200506026
Seelenmeyer, C., Stegmayer, C., Nickel, W.: Unconventional secretion of fibroblast growth factor 2 and galectin-1 does not require shedding of plasma membrane-derived vesicles. FEBS Lett 582(9), 1362–1368 (2008). doi:10.1016/j.febslet.2008.03.024
Ahmad, N., Gabius, H.J., Andre, S., Kaltner, H., Sabesan, S., Roy, R., Liu, B., Macaluso, F., Brewer, C.F.: Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem 279(12), 10841–10847 (2004). doi:10.1074/jbc.M312834200
Earl, L.A., Bi, S., Baum, L.G.: Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology 21(1), 6–12 (2011). doi:10.1093/glycob/cwq144
Nishi, N., Itoh, A., Shoji, H., Miyanaka, H., Nakamura, T.: Galectin-8 and galectin-9 are novel substrates for thrombin. Glycobiology 16(11), 15C–20C (2006). doi:10.1093/glycob/cwl028
Carlsson, S., Oberg, C.T., Carlsson, M.C., Sundin, A., Nilsson, U.J., Smith, D., Cummings, R.D., Almkvist, J., Karlsson, A., Leffler, 12H.: Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology 17(6), 663–676 (2007). doi:10.1093/glycob/cwm026
Patnaik, S.K., Potvin, B., Carlsson, S., Sturm, D., Leffler, H., Stanley, P.: Complex N-glycans are the major ligands for galectin-1, -3, and −8 on Chinese hamster ovary cells. Glycobiology 16(4), 305–317 (2006). doi:10.1093/glycob/cwj063
Merkle, R.K., Cummings, R.D.: Asparagine-linked oligosaccharides containing poly-N-acetyllactosamine chains are preferentially bound by immobilized calf heart agglutinin. J Biol Chem 263(31), 16143–16149 (1988)
Cho, M., Cummings, R.D.: Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem 270(10), 5207–5212 (1995)
Patnaik, S.K., Stanley, P.: Lectin-resistant CHO glycosylation mutants. Methods Enzymol 416, 159–182 (2006). doi:10.1016/S0076-6879(06)16011-5
Andre, S., Kozar, T., Schuberth, R., Unverzagt, C., Kojima, S., Gabius, H.J.: Substitutions in the N-glycan core as regulators of biorecognition: the case of core-fucose and bisecting GlcNAc moieties. Biochemistry 46(23), 6984–6995 (2007). doi:10.1021/bi7000467
Kariya, Y., Kawamura, C., Tabei, T., Gu, J.: Bisecting GlcNAc residues on laminin-332 down-regulate galectin-3-dependent keratinocyte motility. J Biol Chem 285(5), 3330–3340 (2010). doi:10.1074/jbc.M109.038836
Granovsky, M., Fata, J., Pawling, J., Muller, W.J., Khokha, R., Dennis, J.W.: Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med 6(3), 306–312 (2000). doi:10.1038/73163
Dennis, J.W., Laferte, S., Waghorne, C., Breitman, M.L., Kerbel, R.S.: Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236(4801), 582–585 (1987)
Partridge, E.A., Le Roy, C., Di Guglielmo, G.M., Pawling, J., Cheung, P., Granovsky, M., Nabi, I.R., Wrana, J.L., Dennis, J.W.: Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306(5693), 120–124 (2004). doi:10.1126/science.1102109
Lau, K.S., Partridge, E.A., Grigorian, A., Silvescu, C.I., Reinhold, V.N., Demetriou, M., Dennis, J.W.: Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129(1), 123–134 (2007). doi:10.1016/j.cell.2007.01.049
Grigorian, A., Torossian, S., Demetriou, M.: T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol Rev 230(1), 232–246 (2009). doi:10.1111/j.1600-065X.2009.00796.x
Demetriou, M., Granovsky, M., Quaggin, S., Dennis, J.W.: Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409(6821), 733–739 (2001). doi:10.1038/35055582
Ohtsubo, K., Takamatsu, S., Minowa, M.T., Yoshida, A., Takeuchi, M., Marth, J.D.: Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123(7), 1307–1321 (2005). doi:10.1016/j.cell.2005.09.041
Ohtsubo, K., Chen, M.Z., Olefsky, J.M., Marth, J.D.: Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med 17(9), 1067–1075 (2011). doi:10.1038/nm.2414
Markowska, A.I., Jefferies, K.C., Panjwani, N.: Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem 286(34), 29913–29921 (2011). doi:10.1074/jbc.M111.226423
Guo, H.B., Johnson, H., Randolph, M., Lee, I., Pierce, M.: Knockdown of GnT-Va expression inhibits ligand-induced downregulation of the epidermal growth factor receptor and intracellular signaling by inhibiting receptor endocytosis. Glycobiology 19(5), 547–559 (2009). doi:10.1093/glycob/cwp023
Lagana, A., Goetz, J.G., Cheung, P., Raz, A., Dennis, J.W., Nabi, I.R.: Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 26(8), 3181–3193 (2006). doi:10.1128/MCB.26.8.3181-3193.2006
Saravanan, C., Liu, F.T., Gipson, I.K., Panjwani, N.: Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci 122(Pt 20), 3684–3693 (2009). doi:10.1242/jcs.045674
Zhao, Y., Sato, Y., Isaji, T., Fukuda, T., Matsumoto, A., Miyoshi, E., Gu, J., Taniguchi, N.: Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J 275(9), 1939–1948 (2008). doi:10.1111/j.1742-4658.2008.06346.x
Gu, J., Taniguchi, N.: Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adhes Migrat 2(4), 243–245 (2008)
Rebbaa, A., Yamamoto, H., Saito, T., Meuillet, E., Kim, P., Kersey, D.S., Bremer, E.G., Taniguchi, N., Moskal, J.R.: Gene transfection-mediated overexpression of beta1,4-N-acetylglucosamine bisecting oligosaccharides in glioma cell line U373 MG inhibits epidermal growth factor receptor function. J Biol Chem 272(14), 9275–9279 (1997)
Sato, Y., Takahashi, M., Shibukawa, Y., Jain, S.K., Hamaoka, R., Miyagawa, J., Yaginuma, Y., Honke, K., Ishikawa, M., Taniguchi, N.: Overexpression of N-acetylglucosaminyltransferase III enhances the epidermal growth factor-induced phosphorylation of ERK in HeLaS3 cells by up-regulation of the internalization rate of the receptors. J Biol Chem 276(15), 11956–11962 (2001). doi:10.1074/jbc.M008551200
Gu, J., Zhao, Y., Isaji, T., Shibukawa, Y., Ihara, H., Takahashi, M., Ikeda, Y., Miyoshi, E., Honke, K., Taniguchi, N.: Beta1,4-N-Acetylglucosaminyltransferase III down-regulates neurite outgrowth induced by costimulation of epidermal growth factor and integrins through the Ras/ERK signaling pathway in PC12 cells. Glycobiology 14(2), 177–186 (2004). doi:10.1093/glycob/cwh016
Yoshimura, M., Nishikawa, A., Ihara, Y., Taniguchi, S., Taniguchi, N.: Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci U S A 92(19), 8754–8758 (1995)
Sheng, Y., Yoshimura, M., Inoue, S., Oritani, K., Nishiura, T., Yoshida, H., Ogawa, M., Okajima, Y., Matsuzawa, Y., Taniguchi, N.: Remodeling of glycoconjugates on CD44 enhances cell adhesion to hyaluronate, tumor growth and metastasis in B16 melanoma cells expressing beta1,4-N-acetylglucosaminyltransferase III. J Int Cancer 73(6), 850–858 (1997)
Yoshimura, M., Ihara, Y., Ohnishi, A., Ijuhin, N., Nishiura, T., Kanakura, Y., Matsuzawa, Y., Taniguchi, N.: Bisecting N-acetylglucosamine on K562 cells suppresses natural killer cytotoxicity and promotes spleen colonization. Cancer Res 56(2), 412–418 (1996)
Narasimhan, S., Schachter, H., Rajalakshmi, S.: Expression of N-acetylglucosaminyltransferase III in hepatic nodules during rat liver carcinogenesis promoted by orotic acid. J Biol Chem 263(3), 1273–1281 (1988)
Nishikawa, A., Fujii, S., Sugiyama, T., Hayashi, N., Taniguchi, N.: High expression of an N-acetylglucosaminyltransferase III in 3′-methyl DAB-induced hepatoma and ascites hepatoma. Biochem Biophys Res Commun 152(1), 107–112 (1988)
Ekuni, A., Miyoshi, E., Ko, J.H., Noda, K., Kitada, T., Ihara, S., Endo, T., Hino, A., Honke, K., Taniguchi, N.: A glycomic approach to hepatic tumors in N-acetylglucosaminyltransferase III (GnT-III) transgenic mice induced by diethylnitrosamine (DEN): identification of haptoglobin as a target molecule of GnT-III. Free Radic Res 36(8), 827–833 (2002)
Yang, X., Bhaumik, M., Bhattacharyya, R., Gong, S., Rogler, C.E., Stanley, P.: New evidence for an extra-hepatic role of N-acetylglucosaminyltransferase III in the progression of diethylnitrosamine-induced liver tumors in mice. Cancer Res 60(12), 3313–3319 (2000)
Yang, X., Tang, J., Rogler, C.E., Stanley, P.: Reduced hepatocyte proliferation is the basis of retarded liver tumor progression and liver regeneration in mice lacking N-acetylglucosaminyltransferase III. Cancer Res 63(22), 7753–7759 (2003)
Lau, K.S., Dennis, J.W.: N-Glycans in cancer progression. Glycobiology 18(10), 750–760 (2008). doi:10.1093/glycob/cwn071
Guo, H.B., Johnson, H., Randolph, M., Nagy, T., Blalock, R., Pierce, M.: Specific posttranslational modification regulates early events in mammary carcinoma formation. Proc Natl Acad Sci U S A 107(49), 21116–21121 (2010). doi:10.1073/pnas.1013405107
Fernandes, B., Sagman, U., Auger, M., Demetriou, M., Dennis, J.W.: Beta 1–6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res 51(2), 718–723 (1991)
Boscher, C., Dennis, J.W., Nabi, I.R.: Glycosylation, galectins and cellular signaling. Curr Opin Cell Biol 23(4), 383–392 (2011). doi:10.1016/j.ceb.2011.05.001
Eude-Le Parco, I., Gendronneau, G., Dang, T., Delacour, D., Thijssen, V.L., Edelmann, W., Peuchmaur, M., Poirier, F.: Genetic assessment of the importance of galectin-3 in cancer initiation, progression, and dissemination in mice. Glycobiology 19(1), 68–75 (2009). doi:10.1093/glycob/cwn105
Mandal, D.K., Brewer, C.F.: Interactions of concanavalin A with glycoproteins: formation of homogeneous glycoprotein-lectin cross-linked complexes in mixed precipitation systems. Biochemistry 31(50), 12602–12609 (1992)
Castells, A., Gusella, J.F., Ramesh, V., Rustgi, A.K.: A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res 60(11), 2836–2839 (2000)
Iida, A., Kurose, K., Isobe, R., Akiyama, F., Sakamoto, G., Yoshimoto, M., Kasumi, F., Nakamura, Y., Emi, M.: Mapping of a new target region of allelic loss to a 2-cM interval at 22q13.1 in primary breast cancer. Gene Chromosome Canc 21(2), 108–112 (1998)
Nagahata, T., Hirano, A., Utada, Y., Tsuchiya, S., Takahashi, K., Tada, T., Makita, M., Kasumi, F., Akiyama, F., Sakamoto, G., Nakamura, Y., Emi, M.: Correlation of allelic losses and clinicopathological factors in 504 primary breast cancers. Breast cancer 9(3), 208–215 (2002)
Zhang, A., Potvin, B., Zaiman, A., Chen, W., Kumar, R., Phillips, L., Stanley, P.: The gain-of-function Chinese hamster ovary mutant LEC11B expresses one of two Chinese hamster FUT6 genes due to the loss of a negative regulatory factor. J Biol Chem 274(15), 10439–10450 (1999)
Acknowledgments
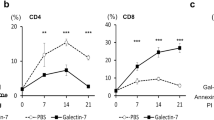
The authors gratefully acknowledge the contribution of Dr. Santosh Patnaik in acquiring the data presented in Fig. 1c. This work was supported by R01 CA30645 and R01 CA36434 to PS, training grant T32 CA009173-34 to HEM, and by resources from the Consortium for Functional Glycomics (Core D and Core H) funded by NIGMS 1U54 GM62116.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miwa, H.E., Song, Y., Alvarez, R. et al. The bisecting GlcNAc in cell growth control and tumor progression. Glycoconj J 29, 609–618 (2012). https://doi.org/10.1007/s10719-012-9373-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9373-6