Abstract
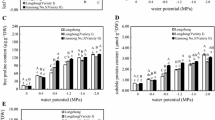
Tall fescue (Festuca arundinacea), a widely planted cool-season turfgrass and forage, is tolerant to heavy metals. However, previous investigation demonstrated that different accessions varied in Pb tolerance. In present study, hydroponic system was used to study the effects of Pb on two tall fescue cultivars, Pb tolerant ‘Silverado’ and Pb sensitive ‘AST7001’, respectively. The results indicated that Pb concentration was 14 times lower in shoots of ‘Silverado’ (1.34 mg g−1 dry weight) versus ‘AST7001’ (19.92 mg g−1 dry weight), although it was higher in roots of ‘Silverado’ (68.28 mg g−1 dry weight) versus ‘AST7001’ (48.7 mg g−1 dry weight), when subjected to 1,000 mg L−1 Pb. In both cultivars, Pb caused an induction in malondialdehyde (MDA) content, to a less increase in ‘Silerado’ than ‘AST7001’. Pb treatment decreased significantly soluble protein content in ‘AST7001’. By contrast, soluble protein content was increased progressively, and the ratio of variable to maximal chlorophyll fluorescence was not affected in ‘Silverado’. Pb treated tall fescue leaves had a greater level of superoxide dismutase (SOD) and guaiacol peroxidase (POD) activity in both cultivars, however, increase was sharp in ‘Silverado’ plants. The results of Q-PCR analysis for genes encoding antioxidant enzyme were in accordance with that of enzyme activities. The higher Pb tolerance of ‘Silverado’ might be attributed to lower shoot Pb concentration and MDA content. Meantime, the amount of soluble protein, activity of SOD and POD, as well as the level of up regulation of Cyt Cu/ZnSOD was all higher in ‘Silverado’ than in ‘AST7001’.






Similar content being viewed by others
References
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Alvarez ME, Lamb C (1997) Oxidative burst-mediated defense responses in plant disease resistance. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Atesi I, Suzen HS, Aydin A, Karakaya A (2004) The oxidative DNA base damage in tests of rats after intraperitoneal cadmium injection. Biometals 17:371–377
Begonia MFT, Begonia GB, Ighoavodha M, Okuyiga-Ezem O, Crudup B (2001) Chelate-induced phytoextraction of lead from contaminated soils using tall fescue (Festuca arundinacea). J Miss Acad Sci 46:15
Begonia MT, Begonia GB, Ighoavodha M, Gilliard D (2005) Lead accumulation by tall fescue (Festuca arundinacea Schreb.) grown on a lead-contaminated soil. Int J Environ Res Public Health 2:228–233
Bian SM, Jiang YW (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hortic-Amsterdam 120:264–270
Boswell FC (1975) Municipal sewage sludge and selected elemental applications to soil: effect on soil and fescue. J Environ Qual 4:267–271
Boucher N, Carpentier R (1999) Hg2+, Cu2+, and Pb2+-induced changes in Photosystem II photochemical yield and energy storage in isolated thylakoid membranes: a study using simultaneous fluorescence and photoacoustis measurements. Photosynth Res 59:167–174
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brooks RR (1998) Phytoarchaeology and hyperaccumulators. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, pp 153–180
Brunet J, Varrault G, Zuily-Fodil Y, Repellin A (2009) Accumulation of lead in the roots of grass pea (Lathyrus sativus L.) plants triggers systemic variation in gene expression in the shoots. Chemosphere 77:1113–1120
Byrne SL, Durandeau K, Nagy I, Barth S (2010) Identification of ABC transporters from Lolium perenne L. that are regulated by toxic levels of selenium. Planta 231:901–911
Chance B, Maehly SK (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Dey SK, Dey J, Patra S, Pothal D (2007) Changes in the antioxidative enzyme activities and lipid peroxidation in wheat seedlings exposed to cadmium and lead stress. Braz J Plant Physiol 19:53–60
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dushenkov V, Kumar PBAN, Motto H, Raskin I (1995) Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol 29:1239–1245
Foito ASL, Byrne SL, Shepherd T, Stewart D, Barth S (2009) Transcriptional and metabolic profiles of Lolium perenne L. genotypes in response to a PEG-induced water stress. Plant Biotechnol J 7:719–732
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
French CJ, Dickinson NM, Putwain PD (2006) Woody biomass phytoremediation of contaminated brownfield land. Environ Pollut 141:387–395
Gajić G, Mitrović M, Pavlović P, Stevanović B, Djurdjević L, Kostić O (2009) An assessment of the tolerance of Ligustrum ovalifolium Hassk. to traffic-generated Pb using physiological and biochemical markers. Ecotoxicol Environ Saf 72:1090–1101
Garza A, Vega R, Soto E (2006) Cellular mechanisms of lead neurotoxicity. Med Sci Monit 12:57–65
Heath RN, Packer H (1968) Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. California Agr Expt Sta Circ 347:1–32
Ibrahim MM, Bafeel SO (2009) Alteration of gene expression, superoxide anion radical and lipid peroxidation induced by lead toxicity in leaves of Lepidium sativum. J Anim Plant Sci 4:281–288
Islam E, Liu D, Li T, Yang X, Jin X, Mahmood Q, Tian S, Li J (2008) Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J Hazard Mater 154:914–926
John R, Ahmad P, Gadgil K, Sharma S (2008) Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant Soil Environ 54:262–270
Kaur G, Singh HP, Batish DR, Kohli RK (2012) Growth, photosynthetic activity and oxidative stress in wheat (Triticum aestivum) after exposure of lead to soil. J Environ Biol 33:265–269
King LD (1981) Effect of swine manure lagoon sludge and municipal sewage sludge on growth, nitrogen recovery, and heavy metal content of fescue grass. J Environ Qual 10:465–472
Kovalchuk I, Titov V, Hohn B, Kovalchuk O (2005) Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat Res 570:149–161
Lamhamdi M, El Galiou O, Bakrim A, Nóvoa-Muñoz JC, Arias-Estévez M, Aarab A, Lafont R (2013) Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J Biol Sci 20:29–36
Lanphear BP, Matte TD, Rogers J, Clickner RP, Dietz B, Bornschein RL, Sucop P, Mahaffey KR, Dixon S, Galke W, Rabinowitz M, Farfel M, Rohde C, Schwartz J, Ashley P, Jacobs DE (1998) The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels: a pooled analysis of 12 epidemiologic studies. Environ Res 79:51–68
Lee JM, Roche JR, Donaghy DJ, Thrush A, Sathish P (2010) Validation of reference genes for quantitative RT-PCR studies of gene expression in perennial ryegrass (Lolium perenne L.). BMC Mol Biol 11:1471–2199
Li HY, Luo HJ, Li DY, Hu T, Fu JM (2012) Antioxidant enzyme activity and gene expression in response to lead stress in perennial ryegrass. J Am Soc Hortic Sci 137:80–85
Li HY, Hu ZR, Fu JM (2014) Lead tolerance and accumulation in different tall fescue genotypes. Pratac Sci 31:1269–1274
Liu JG, Li KQ, Xu JK, Zhang ZJ, Ma TB, Lu XL, Yang JC, Zhu QS (2003) Lead toxicity, uptake, and translocation in different rice cultivars. Plant Sci 165:793–802
Liu W, Shu WS, Lan CY (2004) Viola baoshanensis, a plant that hyperaccumalates cadmium. Sci Bull 49:29–32
Liu JN, Zhou QX, Sun T, Ma LQ, Wang S (2008) Growth responses of three ornamental plants to Cd and Cd-Pb stress and their metal accumulation characteristics. J Hazard Mater 151:261–267
Liu T, Liu SY, Guan H, Ma LG, Chen ZL, Gu HY, Qu LJ (2009) Transcriptional profiling of Arabidopsis seedlings in response to heavy metal lead (Pb). Environ Exp Bot 67:377–386
Liu W, Zhou Q, Zhang Y, Wei S (2010) Lead accumulation in different Chinese cabbage cultivars and screening for pollution-safe cultivars. J Environ Manag 91:781–788
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2001) Phytoremediation of heavy metal-contaminated soils: natural hyperaccumulation versus chemically enhanced phytoextraction. J Environ Qual 30:1919–1926
Lummerzheim M, Sandroni M, Castresana C, De Oliveira D, Van Montagu M, Roby D, Timmerman B (1995) Comparative microscopic and enzymatic characterization of the leaf necrosis induced in Arabidopsis thaliana by lead nitrate and by Xanthomanas campestris pv. Campestris after foliar spray. Plant Cell Environ 18:499–509
Luo H, Li H, Zhang X, Fu J (2011) Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 20:770–778
McKersie BD, Chan Y, de Beus M, Bowley SR, Bowler C, Inzé D, D’Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103:1155–1163
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Mishra S, Srivastava S, Tripathi RD, Kumar R, Seth CS, Gupta DK (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Monteiro MS, Santos C, Soares A, Mann RM (2009) Assessment of biomarkers of cadmium stress in lettuce. Ecotoxicol Environ Saf 72:811–818
Nascimento CWA, Xing B (2006) Phytoextraction: a review on enhanced metal availability and plant accumulation. Sci Agric 63:299–311
Päivöke AEA (2002) Soil lead alters phytase activity and mineral nutrient balance of Pisum sativum. Environ Exp Bot 48:61–73
Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, McCarthy I, del Rio LA (2002) Plant proteases, protein degradation and oxidative stress: role of peroxisomes. Plant Physiol Biochem 40:521–530
Parys E, Romanowska E, Siedlecka M, Poskuta J (1998) The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiol Plant 20:13–322
Prasad TK (1996) Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J 10:1017–1026
Qu RL, Li D, Du R, Qu R (2003) Lead uptake by roots of four turfgrass species in hydroponic cultures. HortScience 38:623–626
Raskin I, Kumar PBA, Dushenkov S, Salt DE (1994) Bioconcentration of heavy metals by plants. Curr Opin Biotechnol 5:285–290
Rastgoo L, Alemzadeh A (2011) Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust J Crop Sci 5:375–383
Reddy A, Kumar S, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Sahi SV, Bryant NL, Sharma NC, Singh SR (2002) Characterization of a lead hyperaccumulator shrub, Sesbania drummondii. Environ Sci Technol 36:4676–4680
Saifullah Meers E, Qadir M, de Caritat P, Tack FM, Du Laing G, Zia MH (2009) EDTA-assisted Pb phytoextraction. Chemosphere 74:1279–1291
Sharma P, Dubey R (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Shu X, Yin LY, Zhang QF, Wang WB (2012) Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ Sci Pollut Res 19:893–902
Singh S, Sinha S (2005) Accumulation of metals and its effects in Brassica juncea (L.) Czern (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicol Environ Saf 62:118–127
Soleimani M, Hajabbasi MA, Afyuni M, Charkhabi AH, Shariatmadari H (2009) Bioaccumulation of nickel and lead by Bermuda grass (Cynodon dactylon) and tall fescue (Festuca arundinacea) from two contaminated soils. CJES 7:59–70
Srivastava AK, Venkatachalam P, Raghothama KG, Sahi SV (2007) Identification of lead-regulated genes by suppression subtractive hybridization in the heavy metal accumulator Sesbania drummondii. Planta 225:1353–1365
Tamura H, Honda M, Sato T, Kamachi H (2005) Pb hyperaccumulation and tolerance in common buckwheat (Fagopyrum esculentum Moench). J Plant Res 118:355–359
Tanaka K, Hibino T, Hayashi Y, Tanaka A, Kishitani S, Takabe T, Yokota S (1999) Salt tolerance of transgenic rice overexpression yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci 148:131–138
Tanhan P, Kruatrachue M, Pokethitiyook P, Chaiyarat R (2007) Uptake and accumulation of cadmium, lead and zinc by Siam weed (Chromolaenaodorata (L.) King & Robinson). Chemosphere 68:323–329
Tsang EWT, Bowler C, Herouart D, Van Camp W, Villarroel R, Genetello C, Van Montagu M, Inze D (1991) Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell 3:783–792
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wang YH, Ying Y, Chen J, Wang XC (2004) Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci 167:671–677
Yang YL, Zhang YY, Wei XL, You J, Wang WR, Lu J, Shi RX (2011) Comparative antioxidative responses and proline metabolism in two wheat cultivars under short term lead stress. Ecotoxicol Environ Saf 74:733–740
Zacchini M, Rea E, Tullio M, de Agazio M (2003) Increased antioxidative capacity in maize calli during and after oxidative stress induced by a long lead treatment. Plant Physiol Biochem 41:49–54
Zeng FR, Mao Y, Cheng WD, Wu FB, Zhang GP (2008) Genotypic and environmental variation in chromium, cadmium and lead concentrations in rice. Environ Pollut 153:309–314
Zhang SS, Zhang HM, Qin R, Jiang WS, Liu DH (2009) Cadmium induction of lipid peroxidation and effects on root tip cells and antioxidant enzyme activities in Vicia faba L. Ecotoxicology 18:814–823
Acknowledgments
This research was funded by the National Natural Science Foundation of China (No. 31201653 and No. 31272194), and Academy–Locality Cooperation Programme (Project No. 2B201131161101616).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, Z., Xie, Y., Jin, G. et al. Growth responses of two tall fescue cultivars to Pb stress and their metal accumulation characteristics. Ecotoxicology 24, 563–572 (2015). https://doi.org/10.1007/s10646-014-1404-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1404-6