Summary
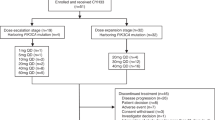
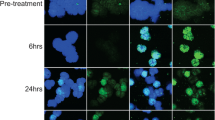
Purpose Triciribine phosphate is a potent, small-molecule inhibitor of activation of all three isoforms of AKT in vitro. AKT is an intracellular protein that, when activated, leads to cellular division; it is dysregulated in a large number of malignancies, and constitutively activating AKT mutations are present in a minority of cancers. Patients and methods In this phase I study triciribine phosphate monohydrate (TCN-PM) was administered to subjects whose tumors displayed evidence of increased AKT phosphorylation (p-AKT) as measured by immunohistochemical analysis (IHC). TCN-PM was administered over 30 min on days 1, 8 and 15 of a 28-day cycle. Tumor biopsy specimens, collected before treatment and on day +15, were assessed for p-AKT by IHC and western blot analyses. Results Nineteen subjects were enrolled; 13 received at least one cycle of therapy, and a total of 34 complete cycles were delivered. One subject was treated at the 45 mg/m2 dose before the study was closed due to its primary objective having been met. No dose-limiting toxic effects were observed. Modest decreases in tumor p-AKT following therapy with TCN-PM were observed at the 35 mg/m2 and 45 mg/m2 dose levels, although definitive conclusions were limited by the small sample size. Conclusions These preliminary data suggest that treatment with TCN-PM inhibits tumor p-AKT at doses that were tolerable. Although single agent activity was not observed in this enriched population, further combination studies of TCN-PM with other signal transduction pathway inhibitors in solid tumors is warranted.


Similar content being viewed by others
References
Bellacosa A, Testa JR, Staal SP et al (1991) A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274–277
Bellacosa A, de Feo D, Godwin AK et al (1995) Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer 64:280–285
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927
Sun M, Wang G, Paciga JE et al (2001) AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol 159:431–437
Cheng JQ, Altomare DA, Klein MA et al (1997) Transforming activity and mitosis-related expression of the AKT2 oncogene: evidence suggesting a link between cell cycle regulation and oncogenesis. Oncogene 12:2793–2801
Hennessy BT, Smith DL, Ram PT et al (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4:988–1004
Schram KH, Townsend LB (1971) The synthesis of 6-amino-4-methyl-8-(β-D-ribofuranosyl (4-H)pyrrolo-[4-3-2depyrimido(4, 5-C) pyridazine, a new tricyclic nucleoside. Tetrahedron Lett 49:4757–4760
Feun LG, Savaraj N, Bodey GP et al (1984) Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res 44:3608–3612
Mittelman A, Casper ES, Godwin TA et al (1983) Phase I study of tricyclic nucleoside phosphate. Cancer Treat Rep 67:159–162
Schilcher RB, Haas CD, Samson MK et al (1986) Phase I evaluation and clinical pharmacology of tricyclic nucleoside 5′-phosphate using a weekly intravenous regimen. Cancer Res 46:3147–3151
Feun LG, Blessing JA, Barrett RJ et al (1993) A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group Study. Am J Clin Oncol 16:506–508
Hoffman K, Holmes FA, Fraschini G et al (1996) Phase I-II study: triciribine (tricyclic nucleoside phosphate) for metastatic breast cancer. Cancer Chemother Pharmacol 37:254–258
O’Connell MJ, Rubin J, Hahn RG et al (1987) Phase II clinical trial of tricyclic nucleoside phosphate for advanced colorectal cancer. Cancer Treat Rep 71:333–334
Yang L, Dan HC, Sun M et al (2004) Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res 64:4394–4399
Powis G, Basseches PJ, Kroschel DM et al (1986) Disposition of tricyclic nucleoside-5′-monophosphate in blood and plasma of patients during phase I and II clinical trials. Cancer Treat Rep 70:359–362
Cancer Therapy Evaluation Program (2003) Common terminology criteria for adverse events, version 3.0. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. March 31
Marshall J, Posey J, Hwang J et al (2007) A phase I trial of RX-0201 (AKT anti-sense) in patients with an advanced cancer. Proc Am Soc Clin Oncol (abstr 3564)
Knowling M, Blackstein M, Tozer R et al (2006) A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: a National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs 24:435–439
Argiris A, Cohen E, Karrison T et al (2006) A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol Ther 5:766–770
Chee KG, Longmate J, Quinn DI et al (2007) The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer 5:433–437
Hedley D, Moore MJ, Hirte H et al (2005) A Phase II trial of perifosine as second line therapy for advanced pancreatic cancer. A study of the Princess Margaret Hospital Phase II Consortium. Proc Am Soc Clin Oncol (abstr 4166)
Ghobrial IM, Leleu X, Rubin N et al (2008) Phase II trial of the novel oral Akt inhibitor perifosine in relapsed and/or refractory Waldenström macroglobulinemia. Proc Am Soc Clin Oncol abstr 8546)
Lindsley CW, Barnett SF, Layton ME et al (2008) The PI3K/Akt pathway: recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Cancer Drug Targets 8:7–18
Yoeli-Lerner M, Yiu GK, Rabinovitz I et al (2005) Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 20:539–550
Hutchinson JN, Jin J, Cardiff RD et al (2004) Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppress tumor invasion. Cancer Res 64:3171–3178
Sawyers CL (2006) Will kinase inhibitors have a dark side? N Engl J Med 20:313–315
Shedden K, Townsend LB, Drach JC et al (2003) A rational approach to personalized anticancer therapy: chemoinformatic analysis reveals mechanistic gene-drug associations. Pharm Res 20:843–847
Acknowledgements
We would like to thank Katherine Stemke Hale, Shrikanth Reddy and Milind Javle for their thoughful review of the manuscript.
Financial disclosures
Dr. Said Sebti is a consultant for Vioquest Pharmaceticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: Vioquest Pharmaceuticals, CA107078 and 1KG02-33967.
Preliminary data from this study were presented at the 16th AACR-NCI-EORTC: Molecular Targets and Cancer Therapeutics Symposium, 2007, San Francisco, California.
Rights and permissions
About this article
Cite this article
Garrett, C.R., Coppola, D., Wenham, R.M. et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs 29, 1381–1389 (2011). https://doi.org/10.1007/s10637-010-9479-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9479-2