Abstract
Background
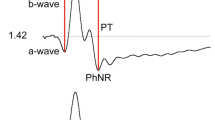
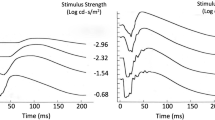
With progressively brighter stimuli, the amplitude of the b-wave of the human photopic electroretinogram (ERG) first increases to a maximal value (V max) and then decreases to finally reach a plateau, a phenomenon known as the photopic hill (PH). A mathematical model combining a Gaussian (G) and a logistic (L) growth function was previously proposed to fit this unusual luminance-response curve, where the G and L functions were suggested to represent, respectively, the OFF and ON retinal pathway contributions to the building of the PH.
Method
The PHs of patients presenting stationary diseases affecting specifically the ON (3 CSNB-1) or OFF (4 CPCPA) retinal pathways as well as patients affected with retinitis pigmentosa (14 RP) of different stages or etiology were analyzed using this mathematical model and compared to the PHs of a group of 28 normal subjects.
Results
The PH of the CSNB-1 patients had a much larger contribution from the G function compared to normal subjects, whereas the opposite was observed for the CPCPA patients. On the other hand, analysis of data from RP patients revealed variable G–L contributions to the building of their PH.
Conclusion
In this study, we confirm the previous claim that the luminance-response function of the photopic ERG b-wave can be decomposed into a Gaussian function and a logistic growth function representing, respectively, the OFF and ON retinal pathways. Furthermore, our findings suggest that this mathematical decomposition could be useful to further segregate and potentially follow the progression of retinopathies such as RP.




Similar content being viewed by others
References
Hamilton R, Bees MA, Chaplin CA, McCulloch DL (2007) The luminance-response function of the human photopic electroretinogram: a mathematical model. Vision Res 47(23):2968–2972
Wali N, Leguire LE (1992) The photopic hill: a new phenomenon of the light adapted electroretinogram. Doc Ophthalmol 80(4):335–345
Wali N, Leguire LE (1993) Fundus pigmentation and the electroretinographic luminance-response function. Doc Ophthalmol 84(1):61–69
Kondo M, Piao CH, Tanikawa A, Horiguchi M, Terasaki H, Miyake Y (2000) Amplitude decrease of photopic ERG b-wave at higher stimulus intensities in humans. Jap J Ophthalmol 44:20–28
Rufiange M, Dassa J, Dembinska O, Koenekoop RK, Little JM, Polomeno RC, Dumont M, Chemtob S, Lachapelle P (2003) The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Res 43:1405–1412
Rufiange M, Dumont M, Lachapelle P (2005) Modulation of the human photopic ERG luminance-response function with the use of chromatic stimuli. Vision Res 45:2321–2330
Rufiange M, Rousseau S, Dembinska O, Lachapelle P (2002) Cone-dominated ERG luminance-response function: the photopic hill revisited. Doc Ophthalmol 104(3):231–248
Ueno S, Kondo M, Niwa S, Terasaki H, Miyake Y (2004) Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040
Garon M-L, Rufiange M, Hamilton R, McCulloch DL, Lachapelle P (2010) Asymetrical growth of the photopic ERG during the light adaptation effect. Doc Ophthalmol 121:177–187
Naka KI, Rushton AH (1966) S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555
Miyake Y, Yagasaki K, Horigushi M, Kawase Y (1987) On- and off-responses in photopic electroretinogram in complete and incomplete types of congenital stationary night blindness. Jap J Ophthalmol 31(1):81–87
Quigley M, Roy MS, Barsoum-Homsy M, Chevrette L, Jacob JL, Milot J (1996–1997) On- and off-responses in the photopic electroretinogram in complete-type congenital stationary night blindness. Doc Ophthalmol 92(3):159–165
Langrová H, Gamer D, Friedburg C, Besch D, Zrenner E, Apfelstedt-Sylla E (2002) Abnormalities of the long flash ERG in congenital stationary night blindness of the Schubert–Bornschein type. Vision Res 42(11):1475–1483
Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A (2000) The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet 26(3):324–327
Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG (2000) Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet 26(3):319–323
Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS (2005) Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci USA 102(13):4884–4889
Zeitz C, Kloeckener-Gruissem B, Forster U, Kohl S, Magyar I, Wissinger B, Mátyás G, Borruat FX, Schorderet DF, Zrenner E, Munier FL, Berger W (2006) Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet 79(4):657–667
Morgans CW, Ren G, Akileswaran L (2006) Localization of nyctalopin in the mammalian retina. Eur J Neurosci 23(5):1163–1171
Slaughter MM, Miller RF (1985) Characterization of an extended glutamate receptor of the on bipolar neuron in the vertebrate retina. J Neurosci 5(1):224–233
Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S (1993) Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem 268(16):11868–11873
Gregg RG, Kamermans M, Klooster J, Lukasiewicz PD, Peachey NS, Vessey KA, McCall MA (2007) Nyctalopin expression in retinal bipolar cells restores visual function in a mouse model of complete X-linked congenital stationary night blindness. J Neurophysiol 98(5):3023–3033
Bahadori R, Biehlmaier O, Zeitz C, Labhart T, Makhankov YV, Forster U, Gesemann M, Berger W, Neuhauss SC (2006) Nyctalopin is essential for synaptic transmission in the cone dominated zebrafish retina. Eur J Neurosci 24(6):1664–1674
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M (2009) ISCEV standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol 118(1):69–77
Lachapelle P, Rousseau S, McKerral M, Benoit J, Polomeno RC, Koenekoop RK, Little JM (1998) Evidence supportive of a functional discrimination between photopic oscillatory potentials as revealed with cone and rod mediated retinopathies. Doc Ophthalmol 95(1):35–54
Heckenlively JR, Martin DA, Rosenbaum AL (1983) Loss of electroretinographic oscillatory potentials, optic atrophy, and dysplasia in congenital stationary night blindness. Am J Ophthalmol 96(4):526–534
Lachapelle P, Little JM, Polomeno RC (1983) The photopic electroretinogram in congenital stationary night blindness with myopia. Invest Ophthalmol Vis Sci 24(4):442–450
Dowling JE (1987) The retina: an approachable part of the brain. The Belknap press of Harvard University Press, Cambridge
Rivolta C, Sharon D, DeAngelis MM, Dryja TP (2002) Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet 11(10):1219–1227
Acknowledgments
This study was supported by the McGill University-Montreal Children’s Hospital Research Institute, the Foundation Fighting Blindness (USA) and FRSQ-Réseau-Vision Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garon, ML., Dorfman, A.L., Racine, J. et al. Estimating ON and OFF contributions to the photopic hill: normative data and clinical applications. Doc Ophthalmol 129, 9–16 (2014). https://doi.org/10.1007/s10633-014-9446-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-014-9446-x