Abstract
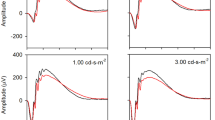
The rod photoreceptor cGMP-gated cation channel has an essential role in phototransduction functioning as the primary point for calcium and sodium entry into the rod outer segment. The channel consists of two subunits, α and β. The α-subunit can function in isolation as an ion channel, and the β-subunit modulates channel activity and has a structural role. We previously reported that a mouse knockout (KO) of the β-subunit and related glutamic acid–rich proteins (GARPs) attenuates rod function and causes structural alterations and slowly progressive retinal degeneration. Here, we have extended our functional analyses of the KO mice evaluating rod and cone function using the electroretinogram in mice up to 4 months of age. Retinal stratification is preserved in the knockout mice at 3 months, and a significant number of cones remain up to 7 months based on PNA staining of cone sheaths. Electroretinography of KO mice at 1 month old revealed a diminished dark-adapted b-wave and normal light-adapted b-wave compared to wild-type mice. Over the next 3 months, both dark- and light-adapted b-wave amplitudes declined, but the reduction was greater for dark-adapted b-wave amplitudes. In one-month-old mice, the critical flicker frequency (CFF) was substantially lower for the KO mice at scotopic intensities, but normal at photopic intensities. CFF values remained stable in the KO mice as the b-wave amplitudes decreased with age. Declining b-wave amplitudes confirm an RP phenotype of rod followed by cone degeneration. Flicker responses show that the cone circuits function normally at threshold despite significant losses in the maximum light-adapted b-wave amplitude. These results confirm that rods are marginally functional in the absence of the β-subunit and in addition show that CFF may be a more sensitive measure of remaining functional cone vision in animal models of RP undergoing progressive rod–cone degeneration.







Similar content being viewed by others
References
Kaupp UB, Seifert R (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82:769–824
Molday RS (1998) Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases: the Friedenwald lecture. Invest Ophthalmol Vis Sci 39:2493–2513
Barnes S (1994) After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience 58:447–459
Hagins WA, Penn RD, Yoshikami S (1970) Dark current and photocurrent in retinal rods. Biophys J 10:380–412
Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223:201–204
Gurevich L, Slaughter MM (1993) Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vis Res 33:2431–2435
Hood DC, Birch DG (1996) Beta wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am A Opt Image Sci Vis 13:623–633
Robson JG, Frishman LJ (1995) Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci 12:837–850
Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB (2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36:881–889
Zhong H, Molday LL, Molday RS, Yau KW (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420:193–198
Zheng J, Trudeau MC, Zagotta WN (2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36:891–896
Korschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, Colville C, Muller F, Dose A, Godde M, Molday L, Kaupp UB, Molday RS (1995) A 240 kDa protein represents the complete beta-subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron 15:627–636
Chen TY, Peng YW, Dhallan RS, Ahamed B, Reed RR, Yau KW (1993) A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature 362:764–767
Kaupp UB, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, Cook NJ, Kangawa K, Matsuo H, Hirose T (1989) Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature 342:762–766
Dhallan RS, Macke JP, Eddy RL, Shows TB, Reed RR, Yau KW, Nathans J (1992) Human rod photoreceptor cGMP-gated channel: amino acid sequence, gene structure, and functional expression. J Neurosci 12:3248–3256
Chen TY, Illing M, Molday LL, Hsu YT, Yau KW, Molday RS (1994) Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Acad Sci USA 91:11757–11761
Zhang Y, Molday LL, Molday RS, Sarfare SS, Woodruff ML, Fain GL, Kraft TW, Pittler SJ (2009) Knockout of GARPs and the beta-subunit of the rod cGMP-gated channel disrupts disk morphogenesis and rod outer segment structural integrity. J Cell Sci 122:1192–1200
Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M, Pfeifer A, Gerstner A, Yau KW, Biel M (2005) Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci 25:130–138
Korschen HG, Beyermann M, Muller F, Heck M, Vantler M, Koch KW, Kellner R, Wolfrum U, Bode C, Hofmann KP, Kaupp UB (1999) Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature 400:761–766
Poetsch A, Molday LL, Molday RS (2001) The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem 276:48009–48016
Pentia DC, Hosier S, Cote RH (2006) The glutamic acid-rich protein-2 (GARP2) is a high affinity rod photoreceptor phosphodiesterase (PDE6)-binding protein that modulates its catalytic properties. J Biol Chem 281:5500–5505
Lyubarsky AL, Chen C, Simon MI, Pugh EN Jr (2000) Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J Neurosci 20:2209–2217
Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, Hurley JB, Baylor DA, Simon MI (1999) Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci USA 96:3718–3722
Nusinowitz S, Nguyen L, Radu R, Kashani Z, Farber D, Danciger M (2003) Electroretinographic evidence for altered phototransduction gain and slowed recovery from photobleaches in albino mice with a MET450 variant in RPE65. Exp Eye Res 77:627–638
Birch DG, Peters AY, Locke KL, Spencer R, Megarity CF, Travis GH (2001) Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4(ABCR) gene. Exp Eye Res 73:877–886
Fain GL, Lisman JE (1999) Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice. Invest Ophthalmol Vis Sci 40:2770–2772
Frasson M, Sahel JA, Fabre M, Simonutti M, Dreyfus H, Picaud S (1999) Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med 5:1183–1187
Lyubarsky AL, Pugh EN Jr (1996) Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci 16:563–571
Lyubarsky AL, Daniele LL, Pugh EN Jr (2004) From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vis Res 44:3235–3251
Birch DG, Fish GE (1987) Rod ERGs in retinitis pigmentosa and cone-rod degeneration. Invest Ophthalmol Vis Sci 28:140–150
Hood DC, Birch DG (1990) A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 5:379–387
Rubin GR, Kraft TW (2007) Flicker assessment of rod and cone function in a model of retinal degeneration. Doc Ophthalmol 115:165–172
Pinilla I, Lund RD, Sauve Y (2005) Cone function studied with flicker electroretinogram during progressive retinal degeneration in RCS rats. Exp Eye Res 80:51–59
Li X, Li W, Dai X, Kong F, Zheng Q, Zhou X, Lü F, Chang B, Rohrer B, Hauswirth WW, Qu J, Pang JJ (2011) Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse: a model for midcourse RPE65 leber congenital amaurosis. Invest Ophthalmol Vis Sci 52:7–15
Rosner B, Glynn RJ, Lee M-LT (2006) Extension of the rank sum test for clustered data: two-group comparisons with group membership defined at the subunit level. Biometrics 62:1251–1259
Toda K, Bush RA, Humphries P, Sieving PA (1999) The electroretinogram of the rhodopsin knockout mouse. Vis Neurosci 16:391–398
Peachey NS, Alexander KR, Fishman GA (1989) The luminance-response function of the dark-adapted human electroretinogram. Vis Res 29:263–270
Herrmann R, Lee B, Arshavsky VY (2011) RGS9 knockout causes a short delay in light responses of ON-bipolar cells. PLoS One 6:e27573
Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M (2001) Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Human Genet 108:328–334
Kondo H, Qin M, Mizota A, Kondo M, Hayashi H, Hayashi K et al (2004) A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest Ophthalmol Vis Sci 45:4433–4439
Michalakis S, Zong X, Becirovic E, Hammelmann V, Wein T, Wanner KT, Biel M (2011) The glutamic acid-rich protein is a gating inhibitor of cyclic nucleotide-gated channels. J Neurosci 31:133–141
Acknowledgments
We thank Dr. Jeffrey Messinger for assistance with visualization and counting of PNA-labeled cone sheaths, Dr. Leigh Millican for assistance with light and electron microscopy, Dr. Chris Strang for help with whole-mount fluorescence microscopy, and Dr. Yuquan Wen and Alex McKeown for assistance with IGOR software analysis. This work was supported by NIH grant EY018143 to SJP. NIH/NEI supported Vision Sciences Core Modules from grant P30 EY03039 were used to facilitate some of the research results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Youwen Zhang and Glen R. Rubin contributed equally.
Rights and permissions
About this article
Cite this article
Zhang, Y., Rubin, G.R., Fineberg, N. et al. Age-related changes in Cngb1-X1 knockout mice: prolonged cone survival. Doc Ophthalmol 124, 163–175 (2012). https://doi.org/10.1007/s10633-012-9317-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-012-9317-2