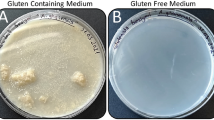
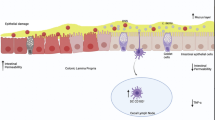
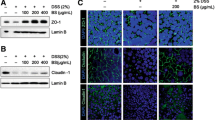
Abstract
Aim
We investigated whether treatment with gliadin induces a paracellular permeability defect that enhances bacterial translocation to mesenteric lymph nodes (MLN) via resident dendritic cells (DC) expressing TLR-2 or 4 in HCD4/HLA-DQ8 transgenic mice.
Methods
HLA-DQ8 transgenic mice were sensitized and subsequently gavaged with gliadin, in the presence or absence of AT1001 (paracellular permeability inhibitor). Non-sensitized mice were gavaged with indomethacin (permeability inducer) or rice cereal. CD11c and CD103 (DC markers) and TLR-2 and 4 were investigated by immunostaining. Intestinal permeability was assessed by paracellular flux of 51Cr-EDTA in Ussing chambers. Bacterial translocation to MLN was performed by plate counting on aerobic and anaerobic conditions.
Results
In gliadin-treated mice, both 51Cr-EDTA flux in jejunal mucosa and aerobic and anaerobic bacterial counts in MLN were increased (p < 0.05) compared to indomethacin-treated mice and controls. The inhibitor AT1001 normalized 51Cr-EDTA flux, but had no effect on bacterial translocation in gliadin-treated mice. In addition, changes in mucosal DC marker distribution such as increased (p < 0.05) trans-epithelial CD103+ cells and reduction (p < 0.05) of CD11c immunostaining were detected in gliadin-treated mice. Moreover, changes in DC markers and TLR-2 or 4 immunophenotypes were not associated.
Conclusions
Pharmacological restoration of paracellular permeability was not sufficient to prevent bacterial translocation in gluten-sensitive mice. We hypothesize that transcellular mechanisms involving CD103+DC and CD11c+DC may explain in gluten-sensitive HCD4/HLA-DQ8 transgenic mice the sustained increased bacterial translocation observed in the absence of a significant inflammatory response.





Similar content being viewed by others
References
Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41–49.
Silva MA, Guiraldes E, Perdue MH. The adverse effect of eating wheat and related grasses for some individuals: coeliac disease and gluten sensitivity. AgroFOOD Ind Hi-Tech. 2007;18:18–20.
Sollid LM, Lie BA. Celiac disease genetics: current concepts and practical applications. Clin Gastroenterol Hepatol. 2005;3:843–851.
Verdú EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594.
Kaukinen K, Collin P, Holm K, Karvonen AL, Pikkarainem P, Mäki M. Small-bowel mucosal inflammation in reticulin or gliadin antibody-positive patients without villous atrophy. Scand J Gastroenterol. 1998;33:944–949.
Niveloni S, et al. Gluten sensitivity in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93:404–408.
Troncone R, et al. Gluten sensitivity in a subset of children with insulin dependent diabetes mellitus. Am J Gastroenterol. 2003;98:590–595.
Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169:5595–5600.
Stepánková R, Tlaskalová-Hogenová H, Sinkora J, Jodl J, Fric P. Changes in jejunal mucosa after long-term feeding of germfree rats with gluten. Scan J Gastroenterol. 1996;31:551–557.
de Kauwe AL, et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol. 2009;182:7440–7450.
Marietta E, et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004;114:1090–1097.
Natividad J. et al. Host responses to intestinal microbial antigens in gluten-sensitive mice. PLoS ONE. 2009;4:e6472.
Pinier M, et al. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology. 2009;136:288–298.
Verdú EF, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G217–G225.
Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520.
Cox MA, Lewis KO, Cooper BT. Measurements of small intestinal permeability markers, lactulose, and mannitol in serum: results in celiac disease. Dig Dis Sci. 1999;44:402–406.
Drago S, et al. Gliadin, zonulin and gut permeability: effect on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419.
Montalto M, Cuoco L, Ricci R, Maggiano N, Vecchio FM, Gasbarrini G. Immunohistochemical analysis of ZO-1 in the duodenal mucosa of patients with untreated and treated celiac disease. Digestion. 2002;65:227–233.
Szakál DN, Gyorffy H, Arató A, et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch. 2010;456:245–250.
Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252.
Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–S60.
Di Sabatino A, et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology. 2007;133:1175–1187.
Qiao SW, et al. Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion. J Immunol. 2004;173:1757–1762.
Ráki M, Tollefsen S, Molberg Ø, Lundin KE, Sollid LM, Jahnsen FL. A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells. Gastroenterology. 2006;131:428–438.
Liu L, MacPherson G. Rat intestinal dendritic cells: immunostimulatory potency and phenotypic characterization. Immunology. 1995;85:88–93.
Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061.
Parker L, Prince L, Sabroe I. Translational mini-review series on Toll-Like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147:199–207.
Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–376.
Szebeni B, et al. Increased mucosal expression of Toll-like receptor (TLR)2 and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr. 2007;45:187–193.
Silva MA, Jury J, Porras M, Vergara P, Perdue MH. Intestinal epithelial barrier dysfunction and dendritic cell redistribution during early stages of inflammation in the rat: role for TLR-2 and -4 Blockage. Inflamm Bowel Dis. 2008;14:632–644.
Hapfelmeier S, et al. The salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–1685.
Sigthorsson G, et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology. 2002;122:1913–1923.
Slack E, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620.
Forsberg G, Fahlgren A, Hörstedt P, Hammarström S, Hernell O, Hammarström ML. Presence of bacteria and innate immunity of intestinal epithelium in childhood celiac disease. Am J Gastroenterol. 2004;99:894–904.
Ashorn S, et al. Serological responses to microbial antigens in celiac disease patients during a gluten-free diet. J Clin Immunol. 2009;29:190–195.
Ashorn S, et al. Elevated serum anti-Saccharomyces cerevisiae, anti-I2 and anti-OmpW antibody levels in patients with suspicion of celiac disease. J Clin Immunol. 2008;28:486–494.
Silva MA. Intestinal dendritic cells and epithelial barrier dysfunction in Crohn’s disease. Inflamm Bowel Dis. 2009;15:436–453.
Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852.
Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367.
Pickard JM, Chervonsky AV. Sampling of the intestinal microbiota by epithelial M cells. Curr Gastroenterol Rep. 2010;5:331–339.
Ciccocioppo R, et al. Effects of gliadin stimulation on bone marrow-derived dendritic cells from HLA-DQ8 transgenic mice. Dig Liver Dis. 2008;40:927–935.
Silva MA, Quera R, Valenzuela J, Salim SY, Söderholm JD, Perdue MH. Dendritic cells and Toll-like receptor 2 and 4 in the ileum from Crohn’s disease patients. Dig Dis Sci. 2008;53:1917–1928.
Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol. 2006;176:2512–2521.
Acknowledgments
This work was supported by grants from the Canadian Association of Gastroenterology (CAG)/Canadian Institute of Health Research (CIHR) (GN2-114709), the Canadian Celiac Association New Investigator Award, ALBA Therapeutics (E. Verdú), the National Institute of Health grant R01 DK048373-13 (A. Fasano), and AGL2008-01440/ALI and Consolider Fun-C-Food CSD2007-00063 from Ministry of Science and Innovation (Spain, Y. Sanz). E. Verdú holds a McMaster University Department of Medicine Internal Career Research Award. Drs. David and Murray were supported in part by R01 DK71003.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Silva, M.A., Jury, J., Sanz, Y. et al. Increased Bacterial Translocation in Gluten-Sensitive Mice Is Independent of Small Intestinal Paracellular Permeability Defect. Dig Dis Sci 57, 38–47 (2012). https://doi.org/10.1007/s10620-011-1847-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1847-z