Abstract
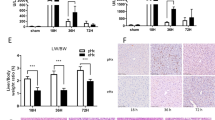
Total parenteral nutrition (TPN) is correlated with progressive liver injury. Such injury may be associated with either an increase or decrease in hepatocyte growth. The goal of these experiments was to determine TPN-related alterations in intrahepatic genes, as they relate with the cell cycle, using microarray techniques. After 7 days of infusion of saline or TPN-solution, hepatocyte gene expression was examined with a 5000-cDNA microarray chip. TPN was associated with an up-regulation of the cyclin kinase Cdc25B mRNA, which controls the cell cycle at the G2/M phase. Based on this, our studies were directed at alterations in genes related to mitosis in this phase of the cell cycle. mRNA expression of mitotic phase inducers and inhibitors were examined. Cdc25B1 mRNA expression increased with TPN. TPN also led to additional significant alterations in the expression of other factors which mediate proliferation in this phase of mitosis. Histologically, TPN resulted in a significant decline in hepatocyte proliferation. Coincident with the alteration in cyclin expression was a significant decrease in hepatocytes in the G2/M phase with TPN administration. This study demonstrates significant alterations in cell cycle gene expression with TPN. The findings correlate with a loss of hepatocyte proliferation and may give insight into the potential mechanism of TPN-induced hepatocyte injury.







Similar content being viewed by others
References
Teitelbaum DH (1997) Parenteral nutrition-associated cholestasis. Curr Opin Pediatr 9:270–275
Karrer F, Bensard D (2000) Neonatal cholestasis. Semin Pediatr Surg 9:166–169
Angelico M, Della Guardia P (2000) Hepatobiliary complications associated with total parenteral nutrition. Aliment Pharmacol Ther 14(Suppl 2):54–57
Chou YH, Yau KI, Hsu HC, Chang MH (1993) Total parenteral nutrition-associated cholestasis in infants: clinical and liver histologic studies. Acta Paediatr Sin 34(4):264–271
Beath SV, Davies P, Papadopoulou A, et al. (1996) Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 31(4):604–606
Mullick FG, Moran CA, Ishak KG (1994) Total parenteral nutrition: a histopathologic analysis of the liver changes in 20 children. Mod Pathol 7(2):190–194
Benjamin DR (1981) Hepatobiliary dysfunction in infants and children associated with long-term total parenteral nutrition. A clinico-pathologic study. Am J Clin Pathol 76(3):276–283
Drongowski RA, Coran AG (1989) An analysis of factors contributing to the development of total parenteral nutrition-induced cholestasis. J Parent Enter Nutr 13(6):586–589
Btaiche IF, Khalidi N (2002) Parenteral nutrition-associated liver complications in children. Pharmacotherapy 22:188– 211
Heubi JE, Daugherty CC (1990) Neonatal cholestasis: an approach for the practicing pediatrician. Curr Probl Pediatr 20(5):233–295
Iyer K, Spitz L, Clayton P (1998) New insight into mechanims of parenteral nutrition - associated cholestasis: role of plant sterols. J Pediatr Surg 33(1):1–6
Aitman T (2001) DNA microarrays in medical practice. Br Med J 323:611
Lockhart D, Winzeler E (2000) Genomics, gene expression and DNA arrays. Nature 405:827–836
Bulera S, Eddy S, Ferguson E, et al. (2001) RNA expression in the early characterization of hepatotoxicants in Wistar rats by high-density DNA microarrays. Hepatology 33:1239–1258
McGaughan G, Shackel N, Gorrell M (2001) Discussion on differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology 121:1263–1269
Shackel N, McGuinness P, Abbott C, et al. (2001) Identification of novel molecules and pathogenic pathways in primary biliary cirrhosis: cDNA array analysis of intrahepatic differential gene expression. Gut 49:565–570
Kiristioglu I, Teitelbaum DH (1998) Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res 79(2):91–96
Russo T, Zambrano N, Esposito F, et al. (1995) A p53-independent pathway for activation of WAF1/CIP1 expression following oxidative stress. J Biol Chem 270:29386–19391
Yang H, Fan Y, Teitelbaum DH (2003) Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol 284(4):G629–G637
Evans W (1970) Fractionation of liver plasma membranes prepared by zonal centrifugation. Biochem J 166:833–842
Doucet J, Trifaro J (1988) A discontinuous and highly porous sodium dodecyl sulfate polyacrylamide slab gel system of high resolution. Anal Biochem 168:265–271
Fraker P, King L, Lill-Elghanian D, Telford W (1995) Quantification of apoptotic events in pure and heterogeneous populations of cells using the flow cytometer. Methods Cell Biol 46:57–76
Goldstein D, Dudoit S, Speed T (2000) Power of a score test for quantitative trait linkage analysis of relative pairs. Genet Epidemiol 19(Suppl 1):S85–S91
Peden V, Witzleben C, Skelton M (1971) Total parenteral nutrition [Letter]. J Pediatr 78:180–181
Sax HC, Bower RH (1988) Hepatic complications of total parenteral nutrition. J Parent and Enter Nutr 12(6):615– 618
Bell R, Ferry G, Smith E (1986) Total parenteral nutrition-associated cholestasis in infants. J Parent Enter Nutr 10:356– 359
Merritt R (1986) Cholestasis associated with total parenteral nutrition. J Pediatr Gastroenterol Nutr 5:9–22
Pines J (1999) Cell cycle. Checkpoint on the nuclear frontier. Nature 397:104–105
Pines J (1999) Four-dimensional control of the cell cycle. Nat Cell Biol 1:E3–E9
Gould K, Nurse P (1989) Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342:39–40
Lammer C, Wagerer S, Saffrich R, et al. (1998) The cdc25B phosphatase is essential for the G2/M phase transition in human cells. J Cell Sci 111:2445–2453
Miyata H, Doki Y, Yamamoto H, et al. (2001) Overexpression of CDC25B overrides radiation-induced G2-M arrest and results in increased apoptosis in esophageal cancer cells. Cancer Res 61:3188–3193
Gasparotto D, Maestro R, Piccinin S, et al. (1997) Overexpression of CDC25A and CDC25B in head and neck cancers. Cancer Res 57:2366–2369
Forrest A, McCormack A, DeSouza C, et al. (1999) Multiple splicing variants of cdc25B regulate G2/M progression. Biochem Biophys Res Commun 260:510–515
Pasternack M Jr, Liu X, Goodman RA, Rannels DE (1997) Regulated stimulation of epithelial cell DNA synthesis by fibroblast-derived mediators. Am J Physiol 272(4, Pt 1):L619–L630
Weinert T (1997) A DNA damage checkpoint meets the cell cycle engine. Science 277:1450–1451
Buchman AL (2003) Choline deficiency during parenteral nutrition in humans. Nutr Clin Pract 18:353–358
Spencer AU, Tracy T, Auothmany M, et al. (2005) Neonatal risk of parenteral nutrition-associated cholestasis: A multivariate analysis of the effect of taurine. J Parent Enter Nutr 29(5):337–344
Staunton M, Phelan DM (1995) Manganese toxicity in a patient with cholestasis receiving total parenteral nutrition [letter; comment]. Anaesthesia 50(7):665
Moss RL, Haynes AL, Pastuszyn A, Glew RH (1999) Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res 45(5, Pt 1):664–668
Bunz F, Dutriaux A, Lengauer C, et al. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501
Chan T, Hwang P, Hermeking H, et al. (2000) Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev 14:1584–1588
De Handt H, Elwi A, Soliman M (1966) Observations of the binucleate cells of the liver. Nature 212:827–829
Chen Y, Wan B (1986) A study on amitosis of the nucleus of the mammalian cell. I. A study under the light and transmission electron microscope. Acta Anat (Basel) 127:69–71
Hennig A, Elias H (1971) Frequency of binuclear liver cells. Verh Anat Ges 66:475–482
Skladanowski A (2002) Modulation of G2 arrest enhances cell death induced by the antitumor 1-nitroacridine derivative, nitracrine. Apoptosis 7:347–359
Strathdee G, Sansom O, Sim A, et al. (2001) A role for mismatch repair in control of DNA ploidy following DNA damage. Oncogene 20:1923–1927
Yan H, Papadopoulos N, Marra G, et al. (2000) Conversion of diploidy to haploidy. Nature 403:723–724
Alverdy JC, Aoys E, Moss GS (1988) Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 104(2):185–190
Acknowledgments
This work was supported by NIH Grant AI44076-01 and also supported in part by the UM-Comprehensive Cancer Center, NIH 5P30CA46592I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tazuke, Y., Wildhaber, B.E., Yang, H. et al. Total Parenteral Nutrition Leads to Alteration of Hepatocyte Cell Cycle Gene Expression and Proliferation in the Mouse. Dig Dis Sci 52, 920–930 (2007). https://doi.org/10.1007/s10620-006-9364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9364-1