Summary
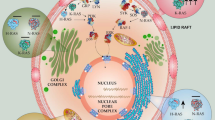
1. Ras signaling and oncogenesis depend on the dynamic interplay of Ras with distinctive plasma membrane (PM) microdomains and various intracellular compartments. Such interaction is dictated by individual elements in the carboxy-terminal domain of the Ras proteins, including a farnesyl isoprenoid group, sequences in the hypervariable region (hvr)-linker, and palmitoyl groups in H/N-Ras isoforms.
2. The farnesyl group acts as a specific recognition unit that interacts with prenyl-binding pockets in galectin-1 (Gal-1), galectin-3 (Gal-3), and cGMP phosphodiesterase δ. This interaction appears to contribute to the prolongation of Ras signals in the PM, the determination of Ras effector usage, and perhaps also the transport of cytoplasmic Ras. Gal-1 promotes H-Ras signaling to Raf at the expense of phosphoinositide 3-kinase (PI3-K) and Ral guanine nucleotide exchange factor (RalGEF), while galectin-3 promotes K-Ras signaling to both Raf and PI3-K.
3. The hvr-linker and the palmitates of H-Ras and N-Ras determine the micro- and macro-localizations of these proteins in the PM and in the Golgi, as well as in ‘rasosomes’, randomly moving nanoparticles that carry palmitoylated Ras proteins and their signal through the cytoplasm.
4. The dynamic compartmentalization of Ras proteins contributes to the spatial organization of Ras signaling, promotes redistribution of Ras, and provides an additional level of selectivity to the signal output of this regulatory GTPase.





Similar content being viewed by others
Abbreviations
- FRAP:
-
fluorescence recovery after photobleaching
- G domain:
-
GTPase domain
- Gal-1:
-
galectin-1
- Gal-3:
-
galectin-3
- GDIs:
-
guanine nucleotide-dissociation inhibitors
- GFP:
-
green fluorescent protein
- hvr:
-
hypervariable region
- PI3-K:
-
phosphoinositide 3-kinase
- PM:
-
plasma membrane
- RalBD:
-
Ral-binding domain of Ral-binding protein 1
- RasGAPs:
-
Ras GTPase-activating proteins
- RalGEFs:
-
Ral guanine nucleotide exchange factors
- RasGEFs:
-
Ras guanine nucleotide exchange factors
- RBD:
-
Ras-binding domain of Raf-1
- TIRF:
-
Total internal reflection fluorescence.
REFERENCES
Adamson, P., Paterson, H. F., and Hall, A. (1992). Intracellular localization of the P21rho proteins. J. Cell Biol. 119:617–627.
An, Y., Shao, Y., Alory, C., Matteson, J., Sakisaka, T., Chen, W., Gibbs, R. A., Wilson, I. A., and Balch, W. E. (2003). Geranylgeranyl switching regulates GDI-Rab GTPase recycling. Structure (Camb.) 11:347–357.
Baker, T. L., Zheng, H., Walker, J., Coloff, J. L., and Buss, J. E. (2003). Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-ras. J. Biol. Chem. 278:19292–19300.
Barbacid, M. (1987). ras genes. Annu. Rev. Biochem. 56:779–827.
Bar-Sagi, D., and Hall, A. (2000). Ras and Rho GTPases: A family reunion. Cell 103:227–238.
Berzat, A. C., Buss, J. E., Chenette, E. J., Weinbaum, C. A., Shutes, A., Der, C. J., Minden, A., and Cox, A. D. (2005). Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J. Biol. Chem. 280:33055–33065.
Bivona, T. G., and Philips, M. R. (2003). Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol. 15:136–142.
Boguski, M. S., and McCormick, F. (1993). Proteins regulating Ras and its relatives. Nature 366:643–654.
Bos, J. L. (1995). p21ras: An oncoprotein functioning in growth factor-induced signal transduction. Eur. J. Cancer 31:1051–1054.
Campbell, S. L., Khosravi-Far, R., Rossman, K. L., Clark, G. J., and Der, C. J. (1998). Increasing complexity of Ras signaling. Oncogene 17:1395–1413.
Casey, P. J., and Seabra, M. C. (1996). Protein prenyltransferases. J. Biol. Chem. 271:5289–5292.
Casey, P. J., Solski, P. A., Der, C. J., and Buss, J. E. (1989). p21ras is modified by a farnesyl isoprenoid. Proc. Natl. Acad. Sci. U.S.A. 86:8323–8327.
Chiu, V. K., Bivona, T., Hach, A., Sajous, J. B., Silletti, J., Wiener, H., Johnson, R. L., 2nd, Cox, A. D., and Philips, M. R. (2002). Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell. Biol. 4:343–350.
Chiu, V. K., Silletti, J., Dinsell, V., Wiener, H., Loukeris, K., Ou, G., Philips, M. R., and Pillinger, M. H. (2004). Carboxyl methylation of Ras regulates membrane targeting and effector engagement. J. Biol. Chem. 279:7346–7352.
Chong, H., Vikis, H. G., and Guan, K. L. (2003). Mechanisms of regulating the Raf kinase family. Cell. Signal. 15:463–469.
Choy, E., Chiu, V. K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I. E., and Philips, M. R. (1999). Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell 98:69–80.
Coleman, R. A., Rao, P., Fogelsong, R. J., and Bardes, E. S. (1992). 2-Bromopalmitoyl-CoA and 2-bromopalmitate: Promiscuous inhibitors of membrane-bound enzymes. Biochim. Biophys. Acta 1125:203–209.
Corbett, K. D., and Alber, T. (2001). The many faces of Ras: Recognition of small GTP-binding proteins. Trends Biochem. Sci. 26:710–716.
Cox, A. D., and Der, C. J. (2003). The dark side of Ras: Regulation of apoptosis. Oncogene 22:8999–9006.
Downward, J. (2003). Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3:11–22.
Du, W., Lebowitz, P. F., and Prendergast, G. C. (1999). Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol. Cell. Biol. 19:1831–1840.
Egozi, Y., Weisz, B., Gana-Weisz, M., Ben-Baruch, G., and Kloog, Y. (1999). Growth inhibition of ras-dependent tumors in nude mice by a potent ras-dislodging antagonist. Int. J. Cancer 80:911–918.
Elad-Sfadia, G., Haklai, R., Ballan, E., Gabius, H. J., and Kloog, Y. (2002). Galectin-1 augments Ras activation and diverts Ras signals to Raf-1 at the expense of phosphoinositide 3-kinase. J. Biol. Chem. 277:37169–37175.
Elad-Sfadia, G., Haklai, R., Balan, E., and Kloog, Y. (2004). Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 279:34922–34930.
Feig, L. A. (2003). Ral-GTPases: Approaching their 15 minutes of fame. Trends Cell Biol. 13:419–425.
Gana-Weisz, M., Halaschek-Wiener, J., Jansen, B., Elad, G., Haklai, R., and Kloog, Y. (2002). The Ras inhibitor S-trans,trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin. Cancer Res. 8:555–565.
Goodwin, J. S., Drake, K. R., Rogers, C., Wright, L., Lippincott-Schwartz, J., Philips, M. R., and Kenworthy, A. K. (2005). Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170:261–272.
Gutierrez, L., Magee, A. I., Marshall, C. J., and Hancock, J. F. (1989). Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 8:1093–1098.
Hancock, J. F. (2003). Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 4:373–384.
Hancock, J. F., Magee, A. I., Childs, J. E., and Marshall, C. J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 57:1167–1177.
Hancock, J. F., and Parton, R. G. (2005). Ras plasma membrane signalling platforms. Biochem. J. 389:1–11.
Hancock, J. F., Paterson, H., and Marshall, C. J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63:133–139.
Hanzal-Bayer, M., Renault, L., Roversi, P., Wittinghofer, A., and Hillig, R. C. (2002). The complex of Arl2-GTP and PDE delta: From structure to function. EMBO J. 21:2095–2106.
Hoffman, G. R., Nassar, N., and Cerione, R. A. (2000). Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100:345–356.
Hu, H., Bliss, J. M., Wang, Y., and Colicelli, J. (2005). RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr. Biol. 15:815–823.
Jaumot, M., Yan, J., Clyde-Smith, J., Sluimer, J., and Hancock, J. F. (2002). The linker domain of the Ha-Ras hypervariable region regulates interactions with exchange factors, Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 277:272–278.
Jiang, X., and Sorkin, A. (2002). Coordinated traffic of Grb2 and Ras during epidermal growth factor receptor endocytosis visualized in living cells. Mol. Biol. Cell. 13:1522–1535.
Kato, K., Cox, A. D., Hisaka, M. M., Graham, S. M., Buss, J. E., and Der, C. J. (1992). Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc. Natl. Acad. Sci. U.S.A. 89:6403–6407.
Kfir, S., Ehrlich, M., Goldshmid, A., Liu, X., Kloog, Y., and Henis, Y. I. (2005). Pathway- and expression level-dependent effects of oncogenic N-Ras: p27(Kip1) mislocalization by the Ral-GEF pathway and Erk-mediated interference with Smad signaling. Mol. Cell. Biol. 25:8239–8250.
Kloog, Y., and Cox, A. D. (2000). RAS inhibitors: Potential for cancer therapeutics. Mol. Med. Today 6:398–402.
Kloog, Y., and Cox, A. D. (2004). Prenyl-binding domains: Potential targets for Ras inhibitors and anti-cancer drugs. Semin. Cancer Biol. 14:253–261.
Kloog, Y., Cox, A. D., and Sinensky, M. (1999). Concepts in Ras-directed therapy. Expert Opin. Invest. Drugs 8:2121–2140.
Linder, M. E., and Deschenes, R. J. (2003). New insights into the mechanisms of protein palmitoylation. Biochemistry 42:4311–4320.
Lobo, S., Greentree, W. K., Linder, M. E., and Deschenes, R. J. (2002). Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277:41268–41273.
Lommerse, P. H., Snaar-Jagalska, B. E., Spaink, H. P., and Schmidt, T. (2005). Single-molecule diffusion measurements of H-Ras at the plasma membrane of live cells reveal microdomain localization upon activation. J. Cell Sci. 118:1799–1809.
Lopez, I., Mak, E. C., Ding, J., Hamm, H. E., and Lomasney, J. W. (2001). A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J. Biol. Chem. 276:2758–2765.
Magee, A. I., Newman, C. M., Giannakouros, T., Hancock, J. F., Fawell, E., and Armstrong, J. (1992). Lipid modifications and function of the ras superfamily of proteins. Biochem. Soc. Trans. 20:497–499.
Magee, A. I., and Seabra, M. C. (2003). Are prenyl groups on proteins sticky fingers or greasy handles? Biochem. J. 376:e3–e4.
Magee, T., and Seabra, M. C. (2005). Fatty acylation and prenylation of proteins: What's hot in fat. Curr. Opin. Cell Biol. 17:190–196.
Malumbres, M., and Barbacid, M. (2003). RAS oncogenes: The first 30 years. Nat. Rev. Cancer 3:459–465.
Marom, M., Haklai, R., Ben-Baruch, G., Marciano, D., Egozi, Y., and Kloog, Y. (1995). Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J. Biol. Chem. 270:22263–22270.
Marshall, C. J. (1993). Protein prenylation: A mediator of protein–protein interactions. Science 259:1865–1866.
Martincic, I., Peralta, M. E., and Ngsee, J. K. (1997). Isolation and characterization of a dual prenylated Rab and VAMP2 receptor. J. Biol. Chem. 272:26991–26998.
Matheny, S. A., Chen, C., Kortum, R. L., Razidlo, G. L., Lewis, R. E., and White, M. A. (2004). Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature 427:256–260.
Meder, D., and Simons, K. (2005). Cell biology. Ras on the roundabout. Science 307:1731–1733.
Michaelson, D., Silletti, J., Murphy, G., D'Eustachio, P., Rush, M., and Philips, M. R. (2001). Differential localization of Rho GTPases in live cells: Regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152:111–126.
Minard, M. E., Kim, L. S., Price, J. E., and Gallick, G. E. (2004). The role of the guanine nucleotide exchange factor Tiam1 in cellular migration, invasion, adhesion and tumor progression. Breast Cancer Res. Treat. 84:21–32.
Mitin, N., Rossman, K. L., and Der, C. J. (2005). Signaling interplay in Ras superfamily function. Curr. Biol. 15:R563–R574.
Murakoshi, H., Iino, R., Kobayashi, T., Fujiwara, T., Ohshima, C., Yoshimura, A., and Kusumi, A. (2004). Single-molecule imaging analysis of Ras activation in living cells. Proc. Natl. Acad. Sci. U.S.A. 101:7317–7322.
Nancy, V., Callebaut, I., El Marjou, A., and de Gunzburg, J. (2002). The delta subunit of retinal rod cGMP phosphodiesterase regulates the membrane association of Ras and Rap GTPases. J. Biol. Chem. 277:15076–15084.
Niv, H., Gutman, O., Henis, Y. I., and Kloog, Y. (1999). Membrane interactions of a constitutively active GFP-Ki-Ras 4B and their role in signaling. Evidence from lateral mobility studies. J. Biol. Chem. 274:1606–1613.
Niv, H., Gutman, O., Kloog, Y., and Henis, Y. I. (2002). Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157:865–872.
Ozaki, Y., Sasagawa, S., and Kuroda, S. (2005). Dynamic characteristics of transient responses. J. Biochem. (Tokyo) 137:659–663.
Paz, A., Haklai, R., Elad-Sfadia, G., Ballan, E., and Kloog, Y. (2001). Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 20:7486–7493.
Pfeffer, S. R. (2001). Rab GTPases: Specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487–491.
Philips, M. R. (2005). Compartmentalized signalling of Ras. Biochem. Soc. Trans. 33:657–661.
Plowman, S. J., and Hancock, J. F. (2005). Ras signaling from plasma membrane and endomembrane microdomains. Biochim. Biophys. Acta 1746:274–283.
Praskova, M., Khoklatchev, A., Ortiz-Vega, S., and Avruch, J. (2004). Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 381:453–462.
Prior, I. A., Muncke, C., Parton, R. G., and Hancock, J. F. (2003). Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 160:165–170.
Rocks, O., Peyker, A., Kahms, M., Verveer, P. J., Koerner, C., Lumbierres, M., Kuhlmann, J., Waldmann, H., Wittinghofer, A., and Bastiaens, P. I. (2005). An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307:1746–1752.
Rotblat, B., Niv, H., Andre, S., Kaltner, H., Gabius, H. J., and Kloog, Y. (2004a). Galectin-1(L11A) predicted from a computed galectin-1 farnesyl-binding pocket selectively inhibits Ras-GTP. Cancer Res. 64:3112–3118.
Rotblat, B., Prior, I. A., Muncke, C., Parton, R. G., Kloog, Y., Henis, Y. I., and Hancock, J. F. (2004b). Three separable domains regulate GTP-dependent association of H-ras with the plasma membrane. Mol. Cell. Biol. 24:6799–6810.
Rotblat, B., Yizhar, O., Haklai, R., Ashery, U., and Kloog, Y. (2006). Ras and its signals traverse the cell on randomly moving nanoparticles. Cancer Res. 66:1974–1981.
Roy, S., Plowman, S., Rotblat, B., Prior, I. A., Muncke, C., Grainger, S., Parton, R. G., Henis, Y. I., Kloog, Y., and Hancock, J. F. (2005). Individual palmitoyl residues serve distinct roles in h-ras trafficking, microlocalization, and signaling. Mol. Cell. Biol. 25:6722–6733.
Scheffzek, K., Ahmadian, M. R., Kabsch, W., Wiesmuller, L., Lautwein, A., Schmitz, F., and Wittinghofer, A. (1997). The Ras–RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277:333–338.
Shalom-Feuerstein, R., Cooks, T., Raz, A., and Kloog, Y. (2005). Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 65:7292–7300.
Shields, J. M., Pruitt, K., McFall, A., Shaub, A., and Der, C. J. (2000). Understanding Ras: ‘it ain't over ‘til it's over’. Trends Cell Biol. 10:147–154.
Sierra, D. A., Popov, S., and Wilkie, T. M. (2000). Regulators of G-protein signaling in receptor complexes. Trends Cardiovasc. Med. 10:263–268.
Silvius, J. R., Bhagatji, P., Leventis, R., and Terrone, D. (2006). K-ras4B and prenylated proteins lacking “second signals” associate dynamically with cellular membranes. Mol. Biol. Cell. 17:192–202.
Smotrys, J. E., and Linder, M. E. (2004). Palmitoylation of intracellular signaling proteins: Regulation and function. Annu. Rev. Biochem. 73:559–587.
Song, S. K., Li, S., Okamoto, T., Quilliam, L. A., Sargiacomo, M., and Lisanti, M. P. (1996). Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 271:9690–9697.
Swarthout, J. T., Lobo, S., Farh, L., Croke, M. R., Greentree, W. K., Deschenes, R. J., and Linder, M. E. (2005). DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. J. Biol. Chem. 280:31141–31148.
Vanhaesebroeck, B., Leevers, S. J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P. C., Woscholski, R., Parker, P. J., and Waterfield, M. D. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70:535–602.
Weisz, B., Giehl, K., Gana-Weisz, M., Egozi, Y., Ben-Baruch, G., Marciano, D., Gierschik, P., and Kloog, Y. (1999). A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene 18:2579–2588.
Wymann, M. P., Sozzani, S., Altruda, F., Mantovani, A., and Hirsch, E. (2000). Lipids on the move: Phosphoinositide 3-kinases in leukocyte function. Immunol. Today 21:260–264.
Zhang, B., Prendergast, G. C., and Fenton, R. G. (2002). Farnesyltransferase inhibitors reverse Ras-mediated inhibition of Fas gene expression. Cancer Res. 62:450–458.
Zimmermann, S., and Moelling, K. (1999). Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741–1744.
ACKNOWLEDGEMENTS
We thank S.R. Smith for editorial assistance. Yoel Kloog is an incumbent of The Jack H. Skirball Chair in Applied Neurobiology. This work was supported in part by grants from The Israel Science Foundation Grants 339/02-3(YK) 424/02-16.6 (UA), the Wolfson Family Foundation Trust (YK), and the Minerva Junior Research Group (UA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashery, U., Yizhar, O., Rotblat, B. et al. Spatiotemporal Organization of Ras Signaling: Rasosomes and the Galectin Switch. Cell Mol Neurobiol 26, 469–493 (2006). https://doi.org/10.1007/s10571-006-9059-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9059-3