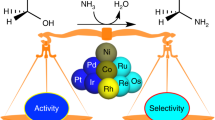
Approaches to control selectivity and activity in the catalytic reductive amination of butyraldehyde with ammonia over carbon supported noble metal catalysts (Ru, Rh, Pd, and Pt) were explored. Detailed analysis of the reaction network shows that the Schiff base N-[butylidene]butan-1-amine is the most prominent initial product and, only after nearly all butyraldehyde had been converted to N-[butylidene]butan-1-amine, amines are detected in the product mixture. From this intermediate, good hydrogenolysis catalysts (Ru, Rh) produce mostly butylamine, while catalysts less active in hydrogenolysis (Pd, Pt) lead to the hydrogenation of N-[butylidene]butan-1-amine to mostly dibutylamine.
Similar content being viewed by others
References
S. Yada, Y. Takagi and M. Hiyamizu, Nippon Kagaku Kaishi, (2) (1995) 107
V.I. Tararov R. Kadyrov T.H. Riermeier A. Börner (2002) Adv. Synth. Catal. 344 IssueID2 200 Occurrence Handle10.1002/1615-4169(200202)344:2<200::AID-ADSC200>3.0.CO;2-4
S. Gomez J.A. Peters T. Maschmeyer (2002) Adv. Synth. Catal. 344 IssueID10 1037 Occurrence Handle10.1002/1615-4169(200212)344:10<1037::AID-ADSC1037>3.0.CO;2-3
L. Marko J. Bakos (1974) J. Organomet. Chem. 81 411 Occurrence Handle10.1016/S0022-328X(00)88210-8
M.V. Klyuev M.L. Khidekel (1980) Transition Met. Chem. 5 134 Occurrence Handle10.1007/BF01396893
T. Gross A.M. Seayad M. Ahmad M. Beller (2002) Org. Lett. 4 IssueID12 2056 Occurrence Handle10.1021/ol0200605
C.A. Willoughby S.L. Buchwald (1992) J. Am. Chem. Soc. 114 IssueID19 7562 Occurrence Handle10.1021/ja00045a038
A. Viso N.E. Lee S.L. Buchwald (1994) J. Am. Chem. Soc. 116 IssueID20 9373 Occurrence Handle10.1021/ja00099a082
M.J. Burk J.P. Martinez J.E. Feaster N. Cosford (1994) Tetrahedron 50 IssueID15 4399 Occurrence Handle10.1016/S0040-4020(01)89375-3
R. Kadyrov T.H. Riermeier U. Dingerdissen V. Tararov A. Börner (2003) J. Org. Chem. 68 4067 Occurrence Handle12737592
Y. Chi Y.-G. Zhou X. Zhang (2003) J. Org. Chem. 68 4120 Occurrence Handle10.1021/jo0353785 Occurrence Handle12737606
M. Freifelder W.D. Smart G.R. Stone (1962) J. Org. Chem. 27 IssueID6 2209
K.A. Pollart R.E. Miller (1962) J. Org Chem. 27 IssueID7 2392
A.L. Bris G. Lefebre F. Coussemant (1964) Bull. Soc. Chim. France 6 1374
A.L. Bris G. Lefebre F. Coussemant (1964) Bull. Soc. Chim. France 7 1584 and 1594
P.L. Mills D.E. Willis R.L. Fenton (1988) Res. Ind. Eng. Chem. 27 IssueID7 1120 Occurrence Handle10.1021/ie00079a006
S. Yada and Y. Takagi, Nippon Kagaku Kaishi, (1) (1991) 20
S. Gomez J.A. Peters J.C. Waal Particlevan der T. Maschmeyer (2003) Appl. Cat. A Gen. 254 77 Occurrence Handle10.1016/S0926-860X(03)00278-3
S. Gomez J.A. Peters J.C. Waal Particlevan der P.J. Brink Particlevan den T. Maschmeyer (2004) Appl. Cat. A Gen. 261 119 Occurrence Handle10.1016/j.apcata.2003.10.037
A.W. Heinen, J.A. Peters, H. van Bekkum, Eur. J. Org. Chem. (13) (2000) 2501
F.B. Siclari, P.P. Rossi, P. Garlasco and M.S. Gaetano, DT 26 47 317 A1, (1977)
D. Kampmann , J. Weber and C. Kniep , EP 0 400 426 A3, (1991)
P.A.M. Grotenhuis, FR 2 656 864 A1, (1991)
C. Kos, E. Artner, E. Kloimstein, F. Hebesberger, R. Haar and E. Lust, DE 43 22 065 A1, (1995)
F. Merger, C.U. Priester, T. Witzel, G. Koppenhoefer and L. Schuster, DE 40 10 252 A1, (1991)
G.Ph. Speranza, J.J. Lin, J.H. Templeton and W.Y. Su, EP 0,388,045 A1, (1990)
R.E. Foster and H.E. Schroeder , US 2,657,240, (1953)
A. Furutani, T. Hibi, M. Yamamoto, K. Tanaka, K. Tada, M. Fukao and G. Suzukamo, EP 0,623,585 A1, (1994)
L.D. Brake , US 3,597,438, (1971)
W.M. Hearon and L.Ch. Fan, US 4,073,804, (1978)
T. Imai, US 4,207,260, (1980)
Ph.F. Jackisch, US 4,521,624, (1985)
F.S. Dovell H. Greenfield (1964) J. Org. Chem. 29 IssueID5 1265
F.S. Dovell H. Greenfield (1965) J. Am. Chem. Soc. 87 IssueID12 2767 Occurrence Handle10.1021/ja01090a050
H. Greenfield F.S. Dovell (1966) J. Org. Chem. 31 IssueID9 3053
The metal dispersion was calculated from the surface atom density of polycrystalline metals given in J.J.F. Scholten, A.P. Pijpers and A.M.L. Hustings, Catal. Rev.-Sci. Eng. (1985) 27(1), 151
The pressure in the autoclave charged with catalyst, ethanol (8 cm3), butyraldehyde (2 cm3) and ammonia (5.5 g) was 12–13 bar at 323 K and 22 bar at 353 K. Pure ammonia has a vapour pressure of 20.3 bar at 323 K and 41.4 bar at 353 K, see E.W. Lemmon, M.O. McLinden and D.G. Friend, in: NIST Chemistry WebBook, NIST Standard Reference Database Number 69, eds. P.J. Linstrom and W.G. Mallard (National Institute of Standards and Technology, Gaithersburg MD, 2003), http://webbook.nist.gov
G.C. Bond (1962) Catalysis by Metals Academic Press London and New York
P.N. Rylander (1967) Catalytic Hydrogenation Over Platinum Metals Academic Press New York and London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bódis, J., Lefferts, L., Müller, T.E. et al. Activity and Selectivity Control in Reductive Amination of Butyraldehyde over Noble Metal Catalysts. Catal Lett 104, 23–28 (2005). https://doi.org/10.1007/s10562-005-7431-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-7431-4