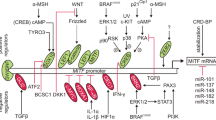
Abstract
The progression of melanoma toward the metastatic phenotype occurs in a defined stepwise manner. While many molecular changes take place early in melanoma development, progression toward the malignant phenotype, most notably during the transition from the radial growth phase (RGP) to the vertical growth phase (VGP) involves deregulated expression of several transcription factors. For example, the switch from RGP to VGP is associated with the loss of the transcription factor AP2α and gain of transcriptional activity of cAMP-responsive element binding protein. Together with the upregulation of microphthalmia-associated transcription factor, activating transcription factor 2, nuclear factor kappa B, and other transcription factors, these changes lead to dysregulated expression or function of important cellular adhesion molecules, matrix degrading enzymes, survival factors, as well as other factors leading to metastatic melanoma. Additionally, recent evidence suggests that microRNAs and RNA editing machinery influence the expression of transcription factors or are regulated themselves by transcription factors. Many of the downstream signaling molecules regulated by transcription factors, such as protease activated receptor-1, interleukin-8, and MCAM/MUC18 represent new treatment prospects.

Similar content being viewed by others
References
Siegel, R., Naishadham, D., & Jemal, A. (2012). Cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 62(1), 10–29.
Balch, C. M., Gershenwald, J. E., Soong, S. J., Thompson, J. F., Atkins, M. B., Byrd, D. R., et al. (2009). Final version of 2009 AJCC melanoma staging and classification. Jounal Clinical Oncology, 27(36), 6199–6206.
Clark, W. H., Jr., Elder, D. E., Guerry, D. T., Epstein, M. N., Greene, M. H., & Van Horn, M. (1984). A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Human Pathology, 15(12), 1147–1165.
Satyamoorthy, K., & Herlyn, M. (2002). Cellular and molecular biology of human melanoma. Cancer Biology & Therapy, 1(1), 14–17.
Leslie, M. C., & Bar-Eli, M. (2005). Regulation of gene expression in melanoma: new approaches for treatment. Journal Cell Biochemistry, 94(1), 25–38.
Greene, V. R., Johnson, M. M., Grimm, E. A., & Ellerhorst, J. A. (2009). Frequencies of NRAS and BRAF mutations increase from the radial to the vertical growth phase in cutaneous melanoma. Journal Investigation Dermatology, 129(6), 1483–1488.
Villanueva, J., & Herlyn, M. (2008). Melanoma and the tumor microenvironment. Current Oncology Reports, 10(5), 439–446.
Chin, L. (2003). The genetics of malignant melanoma: Lessons from mouse and man. Nature Revista Cancer, 3(8), 559–570.
Gray-Schopfer, V., Wellbrock, C., & Marais, R. (2007). Melanoma biology and new targeted therapy. Nature, 445(7130), 851–857.
Leiter, U., Meier, F., Schittek, B., & Garbe, C. (2004). The natural course of cutaneous melanoma. Journal Surgery Oncology, 86(4), 172–178.
Patel, J. K., Didolkar, M. S., Pickren, J. W., & Moore, R. H. (1978). Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. The American Journal of Surgery, 135(6), 807–810.
Fidler, I. J. (2002). Critical determinants of metastasis. Seminars in Cancer Biology, 12(2), 89–96.
Williams, T., Admon, A., Luscher, B., & Tjian, R. (1988). Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Developments, 2(12A), 1557–1569.
Gravel, M., Gao, E., Hervouet-Zeiber, C., Parsons, V., & Braun, P. E. (2000). Transcriptional regulation of 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene expression by cyclic AMP in C6 cells. Journal of Neurochemistry, 75(5), 1940–1950.
Buettner, R., Kannan, P., Imhof, A., Bauer, R., Yim, S. O., Glockshuber, R., et al. (1993). An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Molecular Cell Biology, 13(7), 4174–4185.
Imagawa, M., Chiu, R., & Karin, M. (1987). Transcription factor AP-2 mediates induction by two different signal-transduction pathways: Protein kinase C and cAMP. Cell, 51(2), 251–260.
Schorle, H., Meier, P., Buchert, M., Jaenisch, R., & Mitchell, P. J. (1996). Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature, 381(6579), 235–238.
Luscher, B., Mitchell, P. J., Williams, T., & Tjian, R. (1989). Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes & Development, 3(10), 1507–1517.
Zhang, J., & Williams, T. (2003). Identification and regulation of tissue-specific cis-acting elements associated with the human AP-2alpha gene. Development Dynamics, 228(2), 194–207.
Zeng, Y. X., Somasundaram, K., & el-Deiry, W. S. (1997). AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nature Genetics, 15(1), 78–82.
McPherson, L. A., Loktev, A. V., & Weigel, R. J. (2002). Tumor suppressor activity of AP2alpha mediated through a direct interaction with p53. Journal Biology Chemistry, 277(47), 45028–45033.
Wajapeyee, N., & Somasundaram, K. (2003). Cell cycle arrest and apoptosis induction by activator protein 2alpha (AP-2alpha) and the role of p53 and p21WAF1/CIP1 in AP-2alpha-mediated growth inhibition. Journal Biology Chemistry, 278(52), 52093–52101.
Tellez, C. S., Davis, D. W., Prieto, V. G., Gershenwald, J. E., Johnson, M. M., McCarty, M. F., et al. (2007). Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. Journal Investment Dermatology, 127(2), 387–393.
Berger, A. J., Davis, D. W., Tellez, C., Prieto, V. G., Gershenwald, J. E., Johnson, M. M., et al. (2005). Automated quantitative analysis of activator protein-2alpha subcellular expression in melanoma tissue microarrays correlates with survival prediction. Cancer Research, 65(23), 11185–11192.
Karjalainen, J. M., Kellokoski, J. K., Eskelinen, M. J., Alhava, E. M., & Kosma, V. M. (1998). Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. Journal of Clinical Oncology, 16(11), 3584–3591.
Melnikova, V. O., Dobroff, A. S., Zigler, M., Villares, G. J., Braeuer, R. R., Wang, H., et al. (2010). CREB inhibits AP-2alpha expression to regulate the malignant phenotype of melanoma. PloS One, 5(8), e12452.
Huang, S., Jean, D., Luca, M., Tainsky, M. A., & Bar-Eli, M. (1998). Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. The EMBO Journal, 17(15), 4358–4369.
Gershenwald, J. E., Sumner, W., Calderone, T., Wang, Z., Huang, S., & Bar-Eli, M. (2001). Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene, 20(26), 3363–3375.
Tellez, C., McCarty, M., Ruiz, M., & Bar-Eli, M. (2003). Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. Journal Biology Chemistry, 278(47), 46632–46642.
Jean, D., Gershenwald, J. E., Huang, S., Luca, M., Hudson, M. J., Tainsky, M. A., et al. (1998). Loss of AP-2 results in up-regulation of MCAM/MUC18 and an increase in tumor growth and metastasis of human melanoma cells. Journal Biology Chemistry, 273(26), 16501–16508.
Bar-Eli, M. (1997). Molecular mechanisms of melanoma metastasis. Journal Cell Physiology, 173(2), 275–278.
Ruiz, M., Pettaway, C., Song, R., Stoeltzing, O., Ellis, L., & Bar-Eli, M. (2004). Activator protein 2alpha inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Research, 64(2), 631–638.
Lassam, N., & Bickford, S. (1992). Loss of c-kit expression in cultured melanoma cells. Oncogene, 7(1), 51–56.
Yamamoto, K., Tojo, A., Aoki, N., & Shibuya, M. (1993). Characterization of the promoter region of the human c-kit proto-oncogene. Japan Journal Cancer Research, 84(11), 1136–1144.
Pacifico, M. D., Grover, R., Richman, P. I., Daley, F. M., Buffa, F., & Wilson, G. D. (2005). Development of a tissue array for primary melanoma with long-term follow-up: Discovering melanoma cell adhesion molecule as an important prognostic marker. Plast Reconstruction Surgery, 115(2), 367–375.
Shih, I. M., Elder, D. E., Speicher, D., Johnson, J. P., & Herlyn, M. (1994). Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Research, 54(9), 2514–2520.
Ellis, H. A., & Peart, K. M. (1976). Iliac bone marrow mast cells in relation to the renal osteodystrophy of patients treated by haemodialysis. Journal of Clinical Pathology, 29(6), 502–516.
Xie, S., Luca, M., Huang, S., Gutman, M., Reich, R., Johnson, J. P., et al. (1997). Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Research, 57(11), 2295–2303.
Natali, P. G., Nicotra, M. R., Digiesi, G., Cavaliere, R., Bigotti, A., Trizio, D., et al. (1994). Expression of gp185HER-2 in human cutaneous melanoma: Implications for experimental immunotherapeutics. International Journal of Cancer, 56(3), 341–346.
Melnikova, V. O., Villares, G. J., & Bar-Eli, M. (2008). Emerging roles of PAR-1 and PAFR in melanoma metastasis. Cancer Microenvironment, 1(1), 103–111.
Fischer, E. G., Ruf, W., & Mueller, B. M. (1995). Tissue factor-initiated thrombin generation activates the signaling thrombin receptor on malignant melanoma cells. Cancer Research, 55(8), 1629–1632.
Vu, T. K., Hung, D. T., Wheaton, V. I., & Coughlin, S. R. (1991). Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell, 64(6), 1057–1068.
Macfarlane, S. R., Seatter, M. J., Kanke, T., Hunter, G. D., & Plevin, R. (2001). Proteinase-activated receptors. Pharmacology Review, 53(2), 245–282.
Grand, R. J., Turnell, A. S., & Grabham, P. W. (1996). Cellular consequences of thrombin-receptor activation. Biochemistry Journal, 313(Pt 2), 353–368.
Villares, G. J., Zigler, M., Wang, H., Melnikova, V. O., Wu, H., Friedman, R., et al. (2008). Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Research, 68(21), 9078–9086.
Zucker, S., Conner, C., DiMassmo, B. I., Ende, H., Drews, M., Seiki, M., et al. (1995). Thrombin induces the activation of progelatinase A in vascular endothelial cells. Physiologic regulation of angiogenesis. Journal Biology Chemistry, 270(40), 23730–23738.
Yoshida, E., Verrusio, E. N., Mihara, H., Oh, D., & Kwaan, H. C. (1994). Enhancement of the expression of urokinase-type plasminogen activator from PC-3 human prostate cancer cells by thrombin. Cancer Research, 54(12), 3300–3304.
Melnikova, V. O., Balasubramanian, K., Villares, G. J., Dobroff, A. S., Zigler, M., Wang, H., et al. (2009). Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. Journal Biology Chemistry, 284(42), 28845–28855.
Im, S. Y., Ko, H. M., Kim, J. W., Lee, H. K., Ha, T. Y., Lee, H. B., et al. (1996). Augmentation of tumor metastasis by platelet-activating factor. Cancer Research, 56(11), 2662–2665.
Melnikova, V. O., Mourad-Zeidan, A. A., Lev, D. C., & Bar-Eli, M. (2006). Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. Journal Biology Chemistry, 281(5), 2911–2922.
Axelrad, T. W., Deo, D. D., Ottino, P., Van Kirk, J., Bazan, N. G., Bazan, H. E., et al. (2004). Platelet-activating factor (PAF) induces activation of matrix metalloproteinase 2 activity and vascular endothelial cell invasion and migration. The FASEB Journal, 18(3), 568–570.
Villares, G. J., Dobroff, A. S., Wang, H., Zigler, M., Melnikova, V. O., Huang, L., et al. (2009). Overexpression of protease-activated receptor-1 contributes to melanoma metastasis via regulation of connexin 43. Cancer Research, 69(16), 6730–6737.
Pollmann, M. A., Shao, Q., Laird, D. W., & Sandig, M. (2005). Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Research, 7(4), R522–R534.
el-Sabban, M. E., & Pauli, B. U. (1994). Adhesion-mediated gap junctional communication between lung-metastatatic cancer cells and endothelium. Invasion & Metastasis, 14(1-6), 164–176.
Villares, G. J., Zigler, M., Dobroff, A. S., Wang, H., Song, R., Melnikova, V. O., et al. (2011). Protease activated receptor-1 inhibits the Maspin tumor-suppressor gene to determine the melanoma metastatic phenotype. Processes National Academy Science U S A, 108(2), 626–631.
Bohm, M., Moellmann, G., Cheng, E., Alvarez-Franco, M., Wagner, S., Sassone-Corsi, P., et al. (1995). Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differences, 6(3), 291–302.
Rutberg, S. E., Goldstein, I. M., Yang, Y. M., Stackpole, C. W., & Ronai, Z. (1994). Expression and transcriptional activity of AP-1, CRE, and URE binding proteins in B16 mouse melanoma subclones. Molecular Carcinogenesis, 10(2), 82–87.
Ravnskjaer, K., Kester, H., Liu, Y., Zhang, X., Lee, D., Yates, J. R., 3rd, et al. (2007). Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. The EMBO Journal, 26(12), 2880–2889.
Shaywitz, A. J., & Greenberg, M. E. (1999). CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual Review of Biochemistry, 68, 821–861.
Meyer, T. E., & Habener, J. F. (1993). Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocrine Review, 14(3), 269–290.
Mayr, B., & Montminy, M. (2001). Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature Review Molecular Cell Biology, 2(8), 599–609.
Iourgenko, V., Zhang, W., Mickanin, C., Daly, I., Jiang, C., Hexham, J. M., et al. (2003). Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Process National Academy Science U S A, 100(21), 12147–12152.
Montminy, M. (1997). Transcriptional regulation by cyclic AMP. Annual Review of Biochemistry, 66, 807–822.
Melnikova, V. O., & Bar-Eli, M. (2008). Transcriptional control of the melanoma malignant phenotype. Cancer Biology & Therapy, 7(7), 997–1003.
White, P. C., Shore, A. M., Clement, M., McLaren, J., Soeiro, I., Lam, E. W., et al. (2006). Regulation of cyclin D2 and the cyclin D2 promoter by protein kinase A and CREB in lymphocytes. Oncogene, 25(15), 2170–2180.
Zhang, X., Odom, D. T., Koo, S. H., Conkright, M. D., Canettieri, G., Best, J., et al. (2005). Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Process National Academy Science U S A, 102(12), 4459–4464.
Xie, S., Price, J. E., Luca, M., Jean, D., Ronai, Z., & Bar-Eli, M. (1997). Dominant-negative CREB inhibits tumor growth and metastasis of human melanoma cells. Oncogene, 15(17), 2069–2075.
Jean, D., & Bar-Eli, M. (2000). Regulation of tumor growth and metastasis of human melanoma by the CREB transcription factor family. Molecular Cell Biochemistry, 212(1–2), 19–28.
Jean, D., & Bar-Eli, M. (2001). Targeting the ATF-1/CREB transcription factors by single chain Fv fragment in human melanoma: potential modality for cancer therapy. Criticism Review Immunology, 21(1–3), 275–286.
Jean, D., Tellez, C., Huang, S., Davis, D. W., Bruns, C. J., McConkey, D. J., et al. (2000). Inhibition of tumor growth and metastasis of human melanoma by intracellular anti-ATF-1 single chain Fv fragment. Oncogene, 19(22), 2721–2730.
Jean, D., Harbison, M., McConkey, D. J., Ronai, Z., & Bar-Eli, M. (1998). CREB and its associated proteins act as survival factors for human melanoma cells. Journal Biology Chemistry, 273(38), 24884–24890.
Dobroff, A. S., Wang, H., Melnikova, V. O., Villares, G. J., Zigler, M., Huang, L., et al. (2009). Silencing cAMP-response element-binding protein (CREB) identifies CYR61 as a tumor suppressor gene in melanoma. Journal Biology Chemistry, 284(38), 26194–26206.
Brigstock, D. R. (2003). The CCN family: A new stimulus package. The Journal of Endocrinology, 178(2), 169–175.
Bertolotto, C., Abbe, P., Hemesath, T. J., Bille, K., Fisher, D. E., Ortonne, J. P., et al. (1998). Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. The Journal of Cell Biology, 142(3), 827–835.
Price, E. R., Horstmann, M. A., Wells, A. G., Weilbaecher, K. N., Takemoto, C. M., Landis, M. W., et al. (1998). alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. Journal Biology Chemistry, 273(49), 33042–33047.
Nyormoi, O., & Bar-Eli, M. (2003). Transcriptional regulation of metastasis-related genes in human melanoma. Clinical & Experimental Metastasis, 20(3), 251–263.
Zhuang, L., Lee, C. S., Scolyer, R. A., McCarthy, S. W., Zhang, X. D., Thompson, J. F., et al. (2007). Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Modern Pathology, 20(4), 416–426.
Fuse, N., Yasumoto, K., Suzuki, H., Takahashi, K., & Shibahara, S. (1996). Identification of a melanocyte-type promoter of the microphthalmia-associated transcription factor gene. Biochemistry Biophysics Research Communication, 219(3), 702–707.
Widlund, H. R., & Fisher, D. E. (2003). Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene, 22(20), 3035–3041.
Ganss, R., Schutz, G., & Beermann, F. (1994). The mouse tyrosinase gene. Promoter modulation by positive and negative regulatory elements. Journal Biology Chemistry, 269(47), 29808–29816.
Dorsky, R. I., Moon, R. T., & Raible, D. W. (1998). Control of neural crest cell fate by the Wnt signalling pathway. Nature, 396(6709), 370–373.
McCallion, A. S., & Chakravarti, A. (2001). EDNRB/EDN3 and Hirschsprung disease type II. Pigments Cell Research, 14(3), 161–169.
Lang, D., & Epstein, J. A. (2003). Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Human Molecular Genetics, 12(8), 937–945.
King, R., Googe, P. B., Weilbaecher, K. N., Mihm, M. C., Jr., & Fisher, D. E. (2001). Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. American Journal Surgery Pathology, 25(1), 51–57.
Garraway, L. A., Widlund, H. R., Rubin, M. A., Getz, G., Berger, A. J., Ramaswamy, S., et al. (2005). Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature, 436(7047), 117–122.
Widlund, H. R., Horstmann, M. A., Price, E. R., Cui, J., Lessnick, S. L., Wu, M., et al. (2002). Beta-catenin-induced melanoma growth requires the downstream target microphthalmia-associated transcription factor. The Journal of Cell Biology, 158(6), 1079–1087.
Chien, A. J., Moore, E. C., Lonsdorf, A. S., Kulikauskas, R. M., Rothberg, B. G., Berger, A. J., et al. (2009). Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proceedings National Academy Science U S A, 106(4), 1193–1198.
Takeda, K., Yasumoto, K., Takada, R., Takada, S., Watanabe, K., Udono, T., et al. (2000). Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. Journal Biology Chemistry, 275(19), 14013–14016.
Asundi, J., Reed, C., Arca, J., McCutcheon, K., Ferrando, R., Clark, S., et al. (2011). An antibody–drug conjugate targeting the endothelin B receptor for the treatment of melanoma. Clin Cancer Res, 17(5), 965–975.
Fuchs, S., Amiel, J., Claudel, S., Lyonnet, S., Corvol, P., & Pinet, F. (2001). Functional characterization of three mutations of the endothelin B receptor gene in patients with Hirschsprung’s disease: Evidence for selective loss of Gi coupling. Molecular Medical, 7(2), 115–124.
Imokawa, G., Yada, Y., & Kimura, M. (1996). Signalling mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochemistry Journal, 314(Pt 1), 305–312.
Ji, M., & Andrisani, O. M. (2005). High-level activation of cyclic AMP signaling attenuates bone morphogenetic protein 2-induced sympathoadrenal lineage development and promotes melanogenesis in neural crest cultures. Molecular Cell Biology, 25(12), 5134–5145.
Busca, R., Berra, E., Gaggioli, C., Khaled, M., Bille, K., Marchetti, B., et al. (2005). Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. The Journal of Cell Biology, 170(1), 49–59.
McGill, G. G., Horstmann, M., Widlund, H. R., Du, J., Motyckova, G., Nishimura, E. K., et al. (2002). Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell, 109(6), 707–718.
Du, J., Widlund, H. R., Horstmann, M. A., Ramaswamy, S., Ross, K., Huber, W. E., et al. (2004). Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell, 6(6), 565–576.
Carreira, S., Goodall, J., Denat, L., Rodriguez, M., Nuciforo, P., Hoek, K. S., et al. (2006). Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes & Development, 20(24), 3426–3439.
Jeffs, A. R., Glover, A. C., Slobbe, L. J., Wang, L., He, S., Hazlett, J. A., et al. (2009). A gene expression signature of invasive potential in metastatic melanoma cells. PloS One, 4(12), e8461.
Cheli, Y., Giuliano, S., Botton, T., Rocchi, S., Hofman, V., Hofman, P., et al. (2011). Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene, 30(20), 2307-2318.
Cheli, Y., Giuliano, S., Fenouille, N., Allegra, M., Hofman, V., Hofman, P., et al. (2012). Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene, 31, 2461–2470.
Hai, T. W., Liu, F., Coukos, W. J., & Green, M. R. (1989). Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes & Development, 3(12B), 2083–2090.
Bhoumik, A., Huang, T. G., Ivanov, V., Gangi, L., Qiao, R. F., Woo, S. L., et al. (2002). An ATF2-derived peptide sensitizes melanomas to apoptosis and inhibits their growth and metastasis. Journal Clinical Investment, 110(5), 643–650.
Kaszubska, W., Hooft van Huijsduijnen, R., Ghersa, P., DeRaemy-Schenk, A. M., Chen, B. P., Hai, T., et al. (1993). Cyclic AMP-independent ATF family members interact with NF-kappa B and function in the activation of the E-selectin promoter in response to cytokines. Molecular Cell Biology, 13(11), 7180–7190.
Berger, A. J., Kluger, H. M., Li, N., Kielhorn, E., Halaban, R., Ronai, Z., et al. (2003). Subcellular localization of activating transcription factor 2 in melanoma specimens predicts patient survival. Cancer Research, 63(23), 8103–8107.
Gupta, S., Campbell, D., Derijard, B., & Davis, R. J. (1995). Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science, 267(5196), 389–393.
Kim, S. J., Wagner, S., Liu, F., O’Reilly, M. A., Robbins, P. D., & Green, M. R. (1992). Retinoblastoma gene product activates expression of the human TGF-beta 2 gene through transcription factor ATF-2. Nature, 358(6384), 331–334.
Shimizu, M., Nomura, Y., Suzuki, H., Ichikawa, E., Takeuchi, A., Suzuki, M., et al. (1998). Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Experimental Cell Research, 239(1), 93–103.
Tsai, E. Y., Jain, J., Pesavento, P. A., Rao, A., & Goldfeld, A. E. (1996). Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Molecular Cell Biology, 16(2), 459–467.
Gould Rothberg, B. E., Berger, A. J., Molinaro, A. M., Subtil, A., Krauthammer, M. O., Camp, R. L., et al. (2009). Melanoma prognostic model using tissue microarrays and genetic algorithms. Journal of Clinical Oncology, 27(34), 5772–5780.
Lau, E., Kluger, H., Varsano, T., Lee, K., Scheffler, I., Rimm, D. L., et al. (2012) PKCepsilon promotes oncogenic functions of ATF2 in the nucleus while blocking its apoptotic function at mitochondria. Cell, 148(3), 543–555.
Shah, M., Bhoumik, A., Goel, V., Dewing, A., Breitwieser, W., Kluger, H., et al. (2010). A role for ATF2 in regulating MITF and melanoma development. PLoS Genet, 6(12), e1001258.
Perkins, N. D. (2012). The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer, 12(2), 121–132.
Karin, M., & Greten, F. R. (2005). NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nature Review Immunology, 5(10), 749–759.
Prakash, M., Kale, S., Ghosh, I., Kundu, G. C., & Datta, K. (2011). Hyaluronan-binding protein 1 (HABP1/p32/gC1qR) induces melanoma cell migration and tumor growth by NF-kappa B dependent MMP-2 activation through integrin alpha(v)beta(3) interaction. Cell Signal, 23(10), 1563–1577.
Persad, S., & Dedhar, S. (2003). The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Review, 22(4), 375–384.
Wani, A. A., Jafarnejad, S. M., Zhou, J., & Li, G. (2011). Integrin-linked kinase regulates melanoma angiogenesis by activating NF-kappaB/interleukin-6 signaling pathway. Oncogene, 30(24), 2778–2788.
Feng, Y., Barile, E., De, S. K., Stebbins, J. L., Cortez, A., Aza-Blanc, P., et al. (2011) Effective inhibition of melanoma by BI-69A11 is mediated by dual targeting of the AKT and NF-kappaB pathways. Pigment Cell Melanoma Res, 24(4), 703–713.
Amschler, K., Schon, M. P., Pletz, N., Wallbrecht, K., Erpenbeck, L., & Schon, M. (2010) NF-kappaB inhibition through proteasome inhibition or IKKbeta blockade increases the susceptibility of melanoma cells to cytostatic treatment through distinct pathways. J Invest Dermatol, 130(4), 1073-1086.
Brennecke, J., Stark, A., Russell, R. B., & Cohen, S. M. (2005). Principles of microRNA-target recognition. PLoS Biology, 3(3), e85.
Lee, R. C., Feinbaum, R. L., & Ambros, V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 75(5), 843–854.
Wightman, B., Ha, I., & Ruvkun, G. (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell, 75(5), 855–862.
Krol, J., Loedige, I., & Filipowicz, W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews Genetics, 11(9), 597–610.
Mueller, D. W., & Bosserhoff, A. K. (2009). Role of miRNAs in the progression of malignant melanoma. British Journal of Cancer, 101(4), 551–556.
Griffiths-Jones, S., Saini, H. K., van Dongen, S., & Enright, A. J. (2008). miRBase: tools for microRNA genomics. Nucleic Acids Research, 36(Database issue), D154–D158.
Lu, J., Getz, G., Miska, E. A., Alvarez-Saavedra, E., Lamb, J., Peck, D., et al. (2005). MicroRNA expression profiles classify human cancers. Nature, 435(7043), 834–838.
Gaur, A., Jewell, D. A., Liang, Y., Ridzon, D., Moore, J. H., Chen, C., et al. (2007). Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Research, 67(6), 2456–2468.
Blower, P. E., Verducci, J. S., Lin, S., Zhou, J., Chung, J. H., Dai, Z., et al. (2007). MicroRNA expression profiles for the NCI-60 cancer cell panel. Molecular Cancer Therapy, 6(5), 1483–1491.
Mueller, D. W., Rehli, M., & Bosserhoff, A. K. (2009). miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. Journal Investment Dermatology, 129(7), 1740–1751.
Haflidadottir, B. S., Bergsteinsdottir, K., Praetorius, C., & Steingrimsson, E. (2010). miR-148 regulates Mitf in melanoma cells. PLoS One, 5(7), e11574.
Bemis, L. T., Chen, R., Amato, C. M., Classen, E. H., Robinson, S. E., Coffey, D. G., et al. (2008). MicroRNA-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Research, 68(5), 1362–1368.
Segura, M. F., Hanniford, D., Menendez, S., Reavie, L., Zou, X., Alvarez-Diaz, S., et al. (2009). Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proceeding National Academy Science U S A, 106(6), 1814–1819.
Felicetti, F., Errico, M. C., Bottero, L., Segnalini, P., Stoppacciaro, A., Biffoni, M., et al. (2008). The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Research, 68(8), 2745–2754.
Chen, J., Feilotter, H. E., Pare, G. C., Zhang, X., Pemberton, J. G., Garady, C., et al. (2010). MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. American Journal of Pathology, 176(5), 2520–2529.
Kitago, M., Martinez, S. R., Nakamura, T., Sim, M. S., & Hoon, D. S. (2009). Regulation of RUNX3 tumor suppressor gene expression in cutaneous melanoma. Clinical Cancer Research, 15(9), 2988–2994.
Muller, D. W., & Bosserhoff, A. K. (2008). Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene, 27(52), 6698–6706.
Schultz, J., Lorenz, P., Gross, G., Ibrahim, S., & Kunz, M. (2008). MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Research, 18(5), 549–557.
Bar-Eli, M. (2011). Searching for the ‘melano-miRs’: miR-214 drives melanoma metastasis. The EMBO Journal, 30(10), 1880–1881.
Penna, E., Orso, F., Cimino, D., Tenaglia, E., Lembo, A., Quaglino, E., et al. (2011). microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. The EMBO Journal, 30(10), 1990–2007.
Keegan, L. P., Gallo, A., & O’Connell, M. A. (2001). The many roles of an RNA editor. Nature Review Genet, 2(11), 869–878.
Valente, L., & Nishikura, K. (2005). ADAR gene family and A-to-I RNA editing: diverse roles in posttranscriptional gene regulation. Progress in Nucleic Acid Research and Molecular Biology, 79, 299–338.
Nishikura, K. (2006). Editor meets silencer: crosstalk between RNA editing and RNA interference. Nature Review Molecular Cell Biology, 7(12), 919–931.
Paz, N., Levanon, E. Y., Amariglio, N., Heimberger, A. B., Ram, Z., Constantini, S., et al. (2007). Altered adenosine-to-inosine RNA editing in human cancer. Genome Research, 17(11), 1586–1595.
Maas, S., Patt, S., Schrey, M., & Rich, A. (2001). Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proceeding National Academy Science U S A, 98(25), 14687–14692.
Chapman, P. B., Hauschild, A., Robert, C., Haanen, J. B., Ascierto, P., Larkin, J., et al. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England Journal of Medicine, 364(26), 2507–2516.
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine, 363(8), 711–723.
Robert, C., Thomas, L., Bondarenko, I., O’Day, S., M, D. J., Garbe, C., et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England Journal of Medicine, 364(26), 2517–2526.
Flaherty, K. T., Puzanov, I., Kim, K. B., Ribas, A., McArthur, G. A., Sosman, J. A., et al. Inhibition of mutated, activated BRAF in metastatic melanoma. The New England Journal of Medicine, 363(9), 809-819.
Davies, H., Bignell, G. R., Cox, C., Stephens, P., Edkins, S., Clegg, S., et al. (2002). Mutations of the BRAF gene in human cancer. Nature, 417(6892), 949–954.
Melnikova, V. O., Dobroff, A. S., Zigler, M., Villares, G. J., Braeuer, R. R., Wang, H., et al. (2010). CREB inhibits AP-2alpha expression to regulate the malignant phenotype of melanoma. PLoS One, 5(8), e12452.
Rudolph, D., Tafuri, A., Gass, P., Hammerling, G. J., Arnold, B., & Schutz, G. (1998). Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proceeding National Academy Science U S A, 95(8), 4481–4486.
Yang, E., Boire, A., Agarwal, A., Nguyen, N., O’Callaghan, K., Tu, P., et al. (2009). Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Research, 69(15), 6223–6231.
Becker, R. C., Moliterno, D. J., Jennings, L. K., Pieper, K. S., Pei, J., Niederman, A., et al. (2009). Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet, 373(9667), 919–928.
Mills, L., Tellez, C., Huang, S., Baker, C., McCarty, M., Green, L., et al. (2002). Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Research, 62(17), 5106–5114.
Huang, S., Mills, L., Mian, B., Tellez, C., McCarty, M., Yang, X. D., et al. (2002). Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. The American Journal of Pathology, 161(1), 125–134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mobley, A.K., Braeuer, R.R., Kamiya, T. et al. Driving transcriptional regulators in melanoma metastasis. Cancer Metastasis Rev 31, 621–632 (2012). https://doi.org/10.1007/s10555-012-9358-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-012-9358-8