Abstract
Purpose
Anthracyclines are frequently used in adjuvant treatment for early-stage breast cancer (ESBC). The purpose of this study was to evaluate cardiotoxic effects in the first five years after treatment with different anthracycline-based regimens.
Methods
CCTG MA.21 (NCT000142) was a phase III trial in ESBC that compared cyclophosphamide (75 mg/m2) orally for 14 days, epirubicin (60 mg/m2) and fluorouracil, IV days one and eight (CEF) for six cycles; dose-dense epirubicin (120 mg/m2) and cyclophosphamide, IV every 2 weeks for six cycles with concurrent G-CSF then paclitaxel every 2 weeks for four cycles (ddEC/T); doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks for four cycles then four cycles q3 weekly paclitaxel (175 mg/m2) (AC/T). Endpoints: LVEF decline; LV function changes (heart failure), or Grade 3–4 cardiac ischemia/infarction. A competing risk analysis was performed with endpoints of cardiotoxicity or recurrence in first 5 years after completion of chemotherapy.
Results
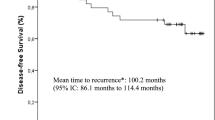
2104 women were randomized. Compliance with cardiac LVEF assessments was 70% at 5 years in all arms. The 5-year cumulative risks of any cardiac event for CEF, ddECT, and AC/T were 22.3% (95%CI 18.9 to 25.7), 14.2% (95%CI 11.0 to 17.3), and 8.1% (95%CI 5.8 to 10.4), respectively, p < 0.0001. At 5 years, women in the ddEC/T and AC/T group had significantly lower risk of cardiotoxicity than those given CEF (HR 0.599 and 0.371, respectively). Most events were asymptomatic drop in LVEF.
Conclusions
Asymptomatic changes in LVEF accounted for most of the cardiotoxicity. The majority of cardiac events occurred in year one although occurrence of cardiotoxicity over time highlights the need for improved risk stratification to guide cardiac surveillance strategies.

Similar content being viewed by others
References
Howlader N, Ries LAG, Mariotto AB et al (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102(20):1584–1598
Jemal A, Ward E, Hao Y et al (2005) Trends in the leading causes of death in the United States, 1970–2002. JAMA 294(10):1255–1259
Jemal A, Ward E, Thun M (2010) Declining death rates reflect progress against cancer. PLoS ONE 5(3):e9584
Miller KD, Siegel RL, Lin CC et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289
Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017 available at www.cancer.ca/Canadian-Cancer-Statistics-2017-EN.pdf August 16, 2017
Bodai BI, Tuso P (2015) Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J 19(2):48–79
Runowicz CD, Leach CR, Henry L et al (2016) American Cancer socieity/American Society of Clinical oncology breast cancer survivorship care guideline. J Clin Oncol 34(6):611–635
Curigliano G, Cardinale D, Dent S et al (2016) Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 66(4):309–325
Suter TM, Ewer MS (2013) Cancer drugs and the heart: importance and management. Eur Heart J 34(15):1102–1111
Aleman BMP, Moser EC, Nuver J et al (2014) Cardiovascular disease after cancer therapy. Eur J Cancer 12(1):18–28
Von Hoff DD, Layard MW, Basa P et al (1979) Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91:710–717
Smith LA, Cornelius VR, Plummer CJ et al (2010) Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10:337
Lotrionte M, Biondi-Zoccai G, Abbate A et al (2013) Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 112(12):1980–1984
Speyer JLGM, Zeleniuch-Jacquotte A, Wernz JC et al (1992) ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol 10:117–127
Coukell AJ, Faulds D (1997) Epirubicin: an updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs. 1997(53):453–482
Piccart-Gebhart M, Proctor M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2—positive breast cancer. NEJM 353:1659–1672
Romond E, Perez E, Bryant J et al (2005) Trastuzumab plus adjuvant chemo therapy for operable HER2—positive breast cancer. NEJM 353:1673–1684
Dennis S, Wolfgang E, Nicholas R et al (2011) Adjuvant trastuzumab in HER2—positive breast cancer. NEJM 365:1273–1283
Joensuu H, Wildiers H, Huovinen R et al (2018) Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2 positive breast cancer: the sold randomized clinical trial. JAMA Oncol 4(9):1199–1206
Citron ML, Berry DA, Cirrincione C et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741/cancer and leukemia group b trial 9741. J Clin Oncol 21:1431–1439
Burnell M, Levine MN, Chapman JW et al (2010) Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol 28:77–78
Gray RJ (1988) A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:p1141–1154
Dignam James S, Qiang Z, Kocherginsky M (2012) The use and interpretation of competing risks regression models. Clin Cancer Res 18(80):2301–2308
Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML (1991) Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266(12):1672–1677
Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, Aktas B, Stefek A, Pollmanns A, Lorenz-Salehi F, Uleer C (2019) West German study planB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol 37(10):799–808
Gray R, Bradley R, Braybrooke J, Liu Z, Peto R, Davies L, Dodwell D, McGale P, Pan H, Taylor C, Barlow W (2019) Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. https://doi.org/10.1016/S0140-6736(18)33137-4
Moja L, Tagliabue L, Balduzzi S et al (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev Issue, 4
Cancer Care Ontario. Ontario Breast Screening Program 2011 Report. Toronto, Canada 2013. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=288834 Accessed 25 July 2017
Armenain AH, Lacchette C, Barac A et al (2017) Prevention and monitoring cardiac dysfunction in survivors of adult cancers: American Society of Clinical oncology clinical practice guideline. J Clin Oncol 25(8):893–911
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, Patel A (2020) Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 31(2):171–190
Acknowledgements
We would to thank all of the women who participated in this trial and the many clinical trials staff that helped to compile these data.
Funding
This work was funded by the Canadian Society Cancer Research Institute (CSCRI), Canadian Institute for Health Research (CIHR), and the National Cancer Institute-US.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Susan Dent—advisory board/consulting, honoraria: Pfizer, Novartis, Eli Lilly; research funding Novartis Kathleen I. Pritchard—Advisory board/consulting, honoraria: Pfizer, Roche, Amgen, Novartis, Eisai, Genomic Health Inc., Myriad Genetic Laboratories.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dent, S.F., Botros, J., Rushton, M. et al. Anthracycline-induced cardiotoxicity in patients with early-stage breast cancer: the Canadian Cancer Trials Group (CCTG) MA.21 experience. Breast Cancer Res Treat 184, 733–741 (2020). https://doi.org/10.1007/s10549-020-05887-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05887-w