Abstract
Background and purpose
Resistance to endocrine therapies in hormone receptor (HR)-positive breast cancer is a significant challenge. Prior studies have shown that low-dose oral cyclophosphamide can transiently deplete regulatory T cells (Tregs) and improve anti-tumor immunity. We investigated the combination of exemestane with cyclophosphamide in patients with advanced HR-positive breast cancer and assessed changes in circulating immune cell subsets.
Methods
This was a single-arm phase II trial of exemestane with cyclophosphamide in patients with metastatic HR-positive/HER2-negative breast cancer who had progressed on prior endocrine therapy (ClinicalTrials.gov: NCT01963481). Primary endpoint was progression-free survival (PFS) at 3 months (RECIST 1.1). Secondary objectives included median PFS, objective response rate, duration of response, and safety. Circulating Tregs (FOXP3+Helios+) and other immune cell subsets were monitored during treatment and compared with healthy controls.
Results
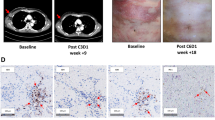
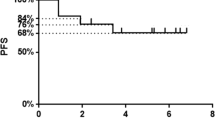
Twenty-three patients were enrolled. Treatment was well tolerated, without grade 4/5 toxicities. Objective responses were seen in 6/23 patients (26.1%; 95% CI 10.2–48.4%) and were durable (median 11.6 months). Three-month PFS rate was 50.1% (95% CI 33.0–76.0%); median PFS was 4.23 months (95% CI 2.8–11.7). No treatment-related decrease in Tregs was observed. However, elevated baseline levels of Naïve Tregs [greater than 2.5 (the median of the naïve Tregs)] were associated with relative risk of disease progression or death [hazard ratio 11.46 (95% CI 2.32–56.5)]. In addition, the baseline levels of Naïve Tregs (adj-p = 0.04), Memory Tregs (adj-p = 0.003), CD4 + Central Memory T cells (adj-p = 0.0004), PD-1 + CD4 + Central Memory T cells (adj-p = 0.008), and PD-1 + CD4 + Effector Memory T cells (adj-p = 0.009) were significantly greater in the patients than in the healthy controls; the baseline levels of %CD4 + Naïve T cells (adj-p = 0.0004) were significantly lower in patients compared with healthy controls (n = 40).
Conclusion
Treg depletion was not observed with low-dose cyclophosphamide when assessed by the specific marker FOXP3 + Helios +; however, baseline naïve Tregs were associated with 3-month PFS. Exemestane/cyclophosphamide combination had favorable safety profile with evidence of clinical activity in heavily pretreated patients.




Similar content being viewed by others
References
Rose C, Vtoraya O, Pluzanska A et al (2003) An open randomised trial of second-line endocrine therapy in advanced breast cancer. Comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer 39:2318–2327
Chia S, Gradishar W, Mauriac L et al (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26:1664–1670
Di Leo A, Jerusalem G, Petruzelka L et al (2010) Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 28:4594–4600
Di Leo A, Jerusalem G, Petruzelka L et al (2014) Final overall survival: fulvestrant 500 mg versus 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 106:337
Thurlimann B, Robertson JF, Nabholtz JM, Buzdar A, Bonneterre J, Arimidex Study G (2003) Efficacy of tamoxifen following anastrozole (‘Arimidex’) compared with anastrozole following tamoxifen as first-line treatment for advanced breast cancer in postmenopausal women. Eur J Cancer 39:2310–2317
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Yardley DA, Noguchi S, Pritchard KI et al (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30:870–884
Topalian SL, Weiner GJ, Pardoll DM (2011) Cancer immunotherapy comes of age. J Clin Oncol 29:4828–4836
Drake CG, Jaffee E, Pardoll DM (2006) Mechanisms of immune evasion by tumors. Adv Immunol 90:51–81
Ghiringhelli F, Menard C, Terme M et al (2005) CD4 + CD25 + regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085
Liu Z, Kim JH, Falo LD Jr, You Z (2009) Tumor regulatory T cells potently abrogate antitumor immunity. J Immunol 182:6160–6167
Nanda R, Chow LQ, Dees EC et al (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol 34:2460–2467
Rugo H, Delord, J-P, Im S-A, et al. (2015) Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, estrogen receptor-positive (ER+)/HER2-negative advanced breast cancer enrolled in KEYNOTE-028. San Antonio Breast Cancer Symposium (SABCS), Abstract S5-07
Adams S, Diamond, JR, Hamilton, EP, et al. (2016) Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). American Society of Clinical Oncology (ASCO) Annual Meeting, Abstract 1009
Adams S LS, Toppmeyer D, et al. (2017) Phase 2 study of pembrolizumab as first-line therapy for PD-L1-positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort. J Clin Oncol 35 (Abstract 1088)
Ghiringhelli F, Larmonier N, Schmitt E et al (2004) CD4 + CD25 + regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34:336–344
Woo EY, Chu CS, Goletz TJ et al (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61:4766–4772
Liyanage UK, Moore TT, Joo HG et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Curiel TJ, Coukos G, Zou L et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H (2005) Inhibition of CD4(+)25 + T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105:2862–2868
Berd D, Mastrangelo MJ (1987) Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res 47:3317–3321
Ghiringhelli F, Menard C, Puig PE et al (2007) Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25 + regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56:641–648
Ge Y, Domschke C, Stoiber N et al (2012) Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother 61:353–362
Dewan MZ, Vanpouille-Box C, Kawashima N et al (2012) Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clin Cancer Res 18:6668–6678
Prieto GA, Rosenstein Y (2006) Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 118:58–65
Polanczyk MJ, Hopke C, Vandenbark AA, Offner H (2006) Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 84:370–378
Benjamini YHY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc 57:289–300
Thome JJ, Grinshpun B, Kumar BV et al (2016) Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol. https://doi.org/10.1126/sciimmunol.aah6506
Cristofanilli M, Turner NC, Bondarenko I et al (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425–439
Baecher-Allan C, Wolf E, Hafler DA (2005) Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4 + CD25 + T cells. Clin Immunol 115:10–18
Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8:523–532
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Bates GJ, Fox SB, Han C et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Walter S, Weinschenk T, Stenzl A et al (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18:1254–1261
Chen G, Gupta R, Petrik S et al (2014) A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res 2:949–961
Greten TF, Ormandy LA, Fikuart A et al (2010) Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4 + T-cell responses in patients with advanced HCC. J Immunother 33:211–218
Lutz ER, Wu AA, Bigelow E et al (2014) Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2:616–631
Audia S, Nicolas A, Cathelin D et al (2007) Increase of CD4 + CD25 + regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4 + CD25 + T lymphocytes. Clin Exp Immunol 150:523–530
Miyara M, Yoshioka Y, Kitoh A et al (2009) Functional delineation and differentiation dynamics of human CD4 + T cells expressing the FoxP3 transcription factor. Immunity 30:899–911
Tran DQ, Ramsey H, Shevach EM (2007) Induction of FOXP3 expression in naive human CD4 + FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110:2983–2990
Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D (2009) Expression of GARP selectively identifies activated human FOXP3 + regulatory T cells. Proc Natl Acad Sci USA 106:13439–13444
Zhou AX, Kozhaya L, Fujii H, Unutmaz D (2013) GARP-TGF-beta complexes negatively regulate regulatory T cell development and maintenance of peripheral CD4 + T cells in vivo. J Immunol 190:5057–5064
Thornton AM, Korty PE, Tran DQ et al (2010) Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3 + T regulatory cells. J Immunol 184:3433–3441
Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK (2013) Helios + and Helios-cells coexist within the natural FOXP3 + T regulatory cell subset in humans. J Immunol 190:2001–2008
Mercer F, Khaitan A, Kozhaya L, Aberg JA, Unutmaz D (2014) Differentiation of IL-17-producing effector and regulatory human T cells from lineage-committed naive precursors. J Immunol 193:1047–1054
Vetizou M, Pitt JM, Daillere R et al (2015) Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350:1079
Beckhove P, Feuerer M, Dolenc M et al (2004) Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Investig 114:67–76
Klebanoff CA, Gattinoni L, Torabi-Parizi P et al (2005) Central memory self/tumor-reactive CD8 + T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA 102:9571–9576
Meng Q, Liu Z, Rangelova E et al (2016) Expansion of tumor-reactive T cells from patients with pancreatic cancer. J Immunother 39:81–89
Liu Z, Meng Q, Bartek J Jr et al (2017) Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology 6:e1252894
Acknowledgements
We thank all the patients who participated in this study and their families, the NYU Clinical Trials Office, and grant support through the Perlmutter Cancer Center (NYUCI Core Grant P30CA016087) (SA, XL, JDG). We also thank Juan Laifalle, PhD, for providing critical reading and guidance for the manuscript.
Funding
Grant support was provided through the Perlmutter Cancer Center (NYUCI Core Grant P30CA016087) (for SA, XL, JDG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwa, M., Li, X., Novik, Y. et al. Serial immunological parameters in a phase II trial of exemestane and low-dose oral cyclophosphamide in advanced hormone receptor-positive breast cancer. Breast Cancer Res Treat 168, 57–67 (2018). https://doi.org/10.1007/s10549-017-4570-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4570-4