Abstract
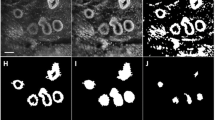
Current techniques for assessing the adequacy of tumour excision during breast conserving surgery do not provide real-time direct cytopathological assessment of the internal cavity walls within the breast. This study investigates the ability of probe-based confocal laser endomicroscopy (pCLE), an emerging imaging tool, to image the morphology of neoplastic and non-neoplastic breast tissues, and determines the ability of histopathologists and surgeons to differentiate these images. Freshly excised tumour samples and adjacent non-diseased sections from 50 consenting patients were stained with 0.01 % acriflavine hydrochloride and imaged using pCLE. All discernible pCLE features were cross-examined with conventional histopathology. Following pattern recognition training, 17 histopathologists and surgeons with no pCLE experience interpreted 50 pCLE images independently whilst blinded to histopathology results. Three-hundred and fifty pCLE image mosaics were analysed. Consistent with histopathology findings, the glandular structures, adipocytes and collagen fibres of normal breast were readily visible on pCLE images. These were distinguishable from the morphological architecture exhibited by invasive and non-invasive carcinoma. The mean accuracy of pCLE image interpretation for histopathologists and surgeons was 94 and 92 %, respectively. Overall, inter-observer agreement for histopathologists was ‘almost perfect’, κ = 0.82; and ‘substantial’ for surgeons, κ = 0.74. pCLE morphological features of neoplastic and non-neoplastic breast tissues are readily visualized and distinguishable with high accuracy by both histopathologists and surgeons. Further research is required to investigate a potential role for the use of pCLE intraoperatively for in situ detection of residual cancerous foci, thereby guiding operating decision-making based on real-time breast cavity scanning.




Similar content being viewed by others
References
Moran M, Schnitt S, Giuliano A, Harris J, Khan S, Horton J, Klimberg S, Chavez-MacGregor M, Freedman G, Houssami N, Johnson P, Morrow M (2014) Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 21(3):704–716. doi:10.1245/s10434-014-3481-4
Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, Bressac C, Spitalier JM (1990) Breast-conserving therapy for macroscopically multiple cancers. Ann Surg 212(1):38–44
Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH (2003) The value of a positive margin for invasive carcinoma in breast-conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys 57(3):724–731
Arriagada R, Le MG, Rochard F, Contesso G, Institute Gustave-Roussy Breast Cancer Group (1996) Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. J Clin Oncol 14(5):1558–1564
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233–1241
Singletary SE (2002) Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 184(5):383–393
Bedwinek JM, Brady L, Perez CA, Goodman R, Kramer S, Grundy G (1980) Irradiation as the primary management of stage I and II adenocarcinoma of the breast: analysis of the RTOG breast registry. Cancer Clin Trials 3(1):11–18
Freedman G, Fowble B, Hanlon A, Nicolaou N, Fein D, Hoffman J, Sigurdson E, Boraas M, Goldstein L (1999) Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 44(5):1005–1015
Singh M, Singh G, Hogan KT, Atkins KA, Schroen AT (2010) The effect of intraoperative specimen inking on lumpectomy re-excision rates. World J Surg Oncol 8:4
Margenthaler JA, Gao F, Klimberg VS (2010) Margin index: a new method for prediction of residual disease after breast-conserving surgery. Ann Surg Oncol 17(10):2696–2701
Balch GC, Mithani SK, Simpson JF, Kelley MC (2005) Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. Am Surg 71(1):22–27 (discussion 27–28)
Jeevan R, Cromwell DA, Trivella M, Lawrence G, Kearins O, Pereira J, Sheppard C, Caddy CM, van der Meulen JH (2012) Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ 345:e4505. doi:10.1136/bmj.e4505
Kreike B, Hart AA, van de Velde T, Borger J, Peterse H, Rutgers E, Bartelink H, van de Vijver MJ (2008) Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys 71(4):1014–1021. doi:10.1016/j.ijrobp.2007.11.029
McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, Engel JM, Onitilo AA (2012) Variability in reexcision following breast conservation surgery. JAMA 307(5):467–475. doi:10.1001/jama.2012.43
Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P (2005) Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol 29(12):1625–1632
Lee CH, Carter D (1995) Detecting residual tumor after excisional biopsy of impalpable breast carcinoma: efficacy of comparing preoperative mammograms with radiographs of the biopsy specimen. AJR 164(1):81–86. doi:10.2214/ajr.164.1.7998574
Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM (2009) Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol 16(10):2717–2730. doi:10.1245/s10434-009-0609-z
Cendan JC, Coco D, Copeland EM 3rd (2005) Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg 201(2):194–198. doi:10.1016/j.jamcollsurg.2005.03.014
Postma EL, Verkooijen HM, van Esser S, Hobbelink MG, van der Schelling GP, Koelemij R, Witkamp AJ, Contant C, van Diest PJ, Willems SM, Borel Rinkes IH, van den Bosch MA, Mali WP, van Hillegersberg R, ROLL Study Group (2012) Efficacy of ‘radioguided occult lesion localisation’ (ROLL) versus ‘wire-guided localisation’ (WGL) in breast conserving surgery for non-palpable breast cancer: a randomised controlled multicentre trial. Breast Cancer Res Treat 136(2):469–478. doi:10.1007/s10549-012-2225-z
Krekel NM, Haloua MH, Lopes Cardozo AM, de Wit RH, Bosch AM, de Widt-Levert LM, Muller S, van der Veen H, Bergers E, de Lange de Klerk ES, Meijer S, van den Tol MP (2013) Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol 14(1):48–54. doi:10.1016/S1470-2045(12)70527-2
Thill M, Roder K, Diedrich K, Dittmer C (2011) Intraoperative assessment of surgical margins during breast conserving surgery of ductal carcinoma in situ by use of radiofrequency spectroscopy. Breast 20(6):579–580. doi:10.1016/j.breast.2011.08.134
Butler-Henderson K, Lee AH, Price RI, Waring K (2014) Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast 23(2):112–119. doi:10.1016/j.breast.2014.01.002
Allweis TM, Kaufman Z, Lelcuk S, Pappo I, Karni T, Schneebaum S, Spector R, Schindel A, Hershko D, Zilberman M, Sayfan J, Berlin Y, Hadary A, Olsha O, Paran H, Gutman M, Carmon M (2008) A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg 196(4):483–489. doi:10.1016/j.amjsurg.2008.06.024
Wallace M, Lauwers GY, Chen Y, Dekker E, Fockens P, Sharma P, Meining A (2011) Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 43(10):882–891. doi:10.1055/s-0030-1256632
Pohl H, Rosch T, Vieth M, Koch M, Becker V, Anders M, Khalifa AC, Meining A (2008) Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut 57(12):1648–1653. doi:10.1136/gut.2008.157461
Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A, Gupta N, Michalek JE, Lauwers GY, Wallace MB (2011) Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 74(3):465–472. doi:10.1016/j.gie.2011.04.004
Loeser CS, Robert ME, Mennone A, Nathanson MH, Jamidar P (2011) Confocal endomicroscopic examination of malignant biliary strictures and histologic correlation with lymphatics. J Clin Gastroenterol 45(3):246–252. doi:10.1097/MCG.0b013e3181fbdc38
Caillol F, Filoche B, Gaidhane M, Kahaleh M (2013) Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris Classification. Dig Dis Sci 58(6):1784–1789. doi:10.1007/s10620-012-2533-5
Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I (2011) First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc 74:1049–1060
Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF (2004) Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127(3):706–713
Kuiper T, van den Broek FJ, van Eeden S, Fockens P, Dekker E (2012) Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol 107(4):543–550. doi:10.1038/ajg.2012.14
Fuchs FS, Zirlik S, Hildner K, Schubert J, Vieth M, Neurath MF (2013) Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur Respir J 41(6):1401–1408. doi:10.1183/09031936.00062512
Sonn GA, Jones SN, Tarin TV, Du CB, Mach KE, Jensen KC, Liao JC (2009) Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J Urol 182(4):1299–1305. doi:10.1016/j.juro.2009.06.039
Li CQ, Yu T, Zuo XL, Xie XJ, Li WB, Chu CL, Zuo F, Li YQ (2011) Effects on confocal laser endomicroscopy image quality by different acriflavine concentrations. J Interv Gastroenterol 1(2):59–63. doi:10.4161/jig.1.2.16828
Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruine A, Masclee A (2010) In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol 8(4):371–378. doi:10.1016/j.cgh.2009.08.006
Gunther U, Daum S, Heller F, Schumann M, Loddenkemper C, Grunbaum M, Zeitz M, Bojarski C (2010) Diagnostic value of confocal endomicroscopy in celiac disease. Endoscopy 42(3):197–202. doi:10.1055/s-0029-1243937
Buchner AM, Gomez V, Heckman MG, Shahid MW, Achem S, Gill KR, Jamil LH, Kahaleh M, Lo SK, Picco M, Riegert-Johnson D, Raimondo M, Sciemeca D, Wolfsen H, Woodward T, Wallace MB (2011) The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc 73(3):556–560. doi:10.1016/j.gie.2011.01.002
Olson TP, Harter J, Munoz A, Mahvi DM, Breslin T (2007) Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol 14(10):2953–2960. doi:10.1245/s10434-007-9437-1
Sumiyoshi K, Nohara T, Iwamoto M, Tanaka S, Kimura K, Takahashi Y, Kurisu Y, Tsuji M, Tanigawa N (2010) Usefulness of intraoperative touch smear cytology in breast-conserving surgery. Exp Ther Med 1(4):641–645. doi:10.3892/etm_00000100
Jorns JM, Visscher D, Sabel M, Breslin T, Healy P, Daignaut S, Myers JL, Wu AJ (2012) Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: one-year experience at an ambulatory surgical center. Am J Clin Pathol 138(5):657–669. doi:10.1309/AJCP4IEMXCJ1GDTS
Weber WP, Engelberger S, Viehl CT, Zanetti-Dallenbach R, Kuster S, Dirnhofer S, Wruk D, Oertli D, Marti WR (2008) Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J Surg 32(12):2599–2606. doi:10.1007/s00268-008-9757-8
Tilli MT, Cabrera MC, Parrish AR, Torre KM, Sidawy MK, Gallagher AL, Makariou E, Polin SA, Liu MC, Furth PA (2007) Real-time imaging and characterization of human breast tissue by reflectance confocal microscopy. J Biomed Opt 12(5):051901. doi:10.1117/1.2799187
Dobbs JL, Ding H, Benveniste AP, Kuerer HM, Krishnamurthy S, Yang W, Richards-Kortum R (2013) Feasibility of confocal fluorescence microscopy for real-time evaluation of neoplasia in fresh human breast tissue. J Biomed Opt 18(10):106016. doi:10.1117/1.JBO.18.10.106016
Dobbs J, Krishnamurthy S, Kyrish M, Benveniste AP, Yang W, Richards-Kortum R (2015) Confocal fluorescence microscopy for rapid evaluation of invasive tumor cellularity of inflammatory breast carcinoma core needle biopsies. Breast Cancer Res Treat 149(1):303–310. doi:10.1007/s10549-014-3182-5
Ragazzi M, Piana S, Longo C, Castagnetti F, Foroni M, Ferrari G, Gardini G, Pellacani G (2014) Fluorescence confocal microscopy for pathologists. Mod Pathol 27(3):460–471. doi:10.1038/modpathol.2013.158
Chang T-P, Palazzo F, Tolley N, Constantinides V, Yang G-Z, Darzi A (2014) Vascularity assessment of parathyroid glands using confocal endomicroscopy: towards an intraoperative imaging tool for real-time in situ viability assessment. Eur J Surg Oncol 40:S3. doi:10.1016/j.ejso.2014.07.008
Simaiaki V, Giataganas P, Guang-Zhong Y (2013) Robot assisted endomicroscopic image mosaicing with optimal surface coverage and reconstruction. In: IEEE 10th international symposium on biomedical imaging (ISBI). 7–11 April 2013. pp 1432–1435. doi:10.1109/ISBI.2013.6556803
Giataganas P, Vitiello V, Simaiaki V, Lopez E, Guang-Zhong Y (2013) Cooperative in situ microscopic scanning and simultaneous tissue surface reconstruction using a compliant robotic manipulator. In: IEEE international conference on robotics and automation (ICRA), 2013. 6–10 May 2013. pp 5378–5383. doi:10.1109/ICRA.2013.6631348
Zuo S, Hughes M, Seneci C, Chang TP, Yang GZ (2015) Towards intraoperative breast endomicroscopy with a novel surface scanning device. IEEE Trans Biomed Eng. doi:10.1109/TBME.2015.2455597
Giataganas P, Hughes M, Yang G-Z (2015) Force adaptive robotically assisted endomicroscopy for intraoperative tumour identification. Int J CARS 10(6):825–832. doi:10.1007/s11548-015-1179-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tou Pin Chang, Daniel R Leff, Sami Shousha, Dimitri J Hadjiminas, Rathi Ramakrishnan, Michael Hughes, Guang-Zhong Yang and Ara Darzi have no conflicts of interest or financial ties to declare.
Rights and permissions
About this article
Cite this article
Chang, T., Leff, D.R., Shousha, S. et al. Imaging breast cancer morphology using probe-based confocal laser endomicroscopy: towards a real-time intraoperative imaging tool for cavity scanning. Breast Cancer Res Treat 153, 299–310 (2015). https://doi.org/10.1007/s10549-015-3543-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3543-8