Abstract
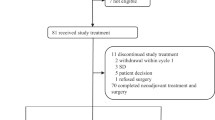
The purpose of this study is to evaluate the efficacy and safety of neoadjuvant treatment with carboplatin and eribulin in patients with early-stage triple negative breast cancer (TNBC), and to explore biomarkers based on DNA and protein expression profiles as predictors of response. Patients with histologically confirmed early-stage TNBC received carboplatin AUC 6 iv every 21 days, and eribulin 1.4 mg/m2 day 1 and day 8 every 21 days for four cycles. The primary endpoint of the study was pathologic complete response (pCR), with secondary endpoints including clinical response and safety of the combination. Exploratory studies assessed DNA-based biomarkers [homologous recombination deficiency (HRD) score, and HR deficiency status (HRD score + BRCA1/BRCA2 mutation status)], protein-based biomarkers (Ki67, TP53, androgen receptor, Cyclin E, CDK2, Cyclin D, CDK4, Pin1 and Smad3), and clinical pretreatment factors as predictors of pCR. 13/30 (43.3 %) patients enrolled in the study achieved pCR. 24 (80.0 %) had a clinical complete or partial response. The combination was safe with mostly grade 1 and 2 toxicities. HRD score (P = 0.0024) and HR deficiency status (P = 0.0012) significantly predicted pCR. Pretreatment cytoplasmic CDK2 was also associated with pCR (P = 0.021). Significant differences in pre- versus post-treatment expression levels of nuclear Cyclin D (P = 0.020), nuclear CDK4 (P = 0.0030), and nuclear Smad3 (P = 0.015) were detected. The combination of carboplatin and eribulin is safe and efficacious in the treatment of early-stage TNBC. HRD score, HR deficiency status, and cytoplasmic CDK2 predicted pCR in this patient population.



Similar content being viewed by others
References
Reis-Filho JS, Tutt AN (2008) Triple negative tumours: a critical review. Histopathology 52:108–118
Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, Imholz AL et al (2013) Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer 108:2172–2177
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13–21
von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15:747–756
Telli ML, Jensen KC, Vinayak S, Kurian AW, Lipson JA, Flaherty P, et al. (2015) A phase II study of gemcitabine, carboplatin and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability (PrECOG 0105). J Clin Oncol. doi:10.1200/JCO.2014.57.0085
Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA et al (2010) Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49:1331–1337
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K et al (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923
Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA et al (2014) Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 148:553–561
Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag K, et al. (2015) TBCRC009: A multi-center Phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. doi:10.1200/JCO.2014.57.6660
Telli ML, Jensen KC, Abkevich V, Hartman A-R, Vinayak S, Lanchbury JS et al (2012) Homologous recombination deficiency (HRD) score predicts pathologic response following neoadjuvant platinum-based therapy in triple-negative and BRCA1/2 mutation-associated breast cancer (BC)(PD09-04). Cancer Res 72(24 suppl):141s
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R et al (2012) Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov 2:366–375
Popova T, Manie E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T et al (2012) Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res 72:5454–5462
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA et al (2012) Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer 107:1776–1782
Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J et al (2010) A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res 16:6159–6168
Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B et al (2014) Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 16:475
Nakagawa M, Bando Y, Nagao T, Morimoto M, Takai C, Ohnishi T et al (2011) Expression of p53, Ki-67, E-cadherin, N-cadherin and TOP2A in triple-negative breast cancer. Anticancer Res 31:2389–2393
Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH et al (2011) Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 13:R22
Tsutsumi Y (2012) Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol 42:375–386
Tarasewicz E, Rivas L, Hamdan R, Dokic D, Parimi V, Bernabe BP et al (2014) Inhibition of CDK-mediated phosphorylation of Smad3 results in decreased oncogenesis in triple negative breast cancer cells. Cell Cycle 13:3191–3201
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Tarasewicz E, Hamdan R, Straehla J, Hardy A, Nunez LO, Zelivianski S et al (2014) CDK4 inhibition and doxorubicin mediate breast cancer cell apoptosis through Smad3 and survivin. Cancer Biol Ther 15:1301–1311
Tarasewicz E, Jeruss JS (2012) Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle 11:2443–2451
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2014
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414–4422
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q et al (2010) Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 28:1145–1153
Cannistra SA (2009) Phase II trials in journal of clinical oncology. J Clin Oncol 27:3073–3076
Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Wanders J et al (2014) A phase III, open-label, randomized, multicenter study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes (S6–7). Cancer Res 72(24 suppl):109s
Twelves C, Akerele C, Wanders J, Cortes J (2010) Eribulin mesylate (E7389) vs treatment of physician’s choice (TPC) in patients (PTS) in patients with metastatic breast cancer (MBC): subgroup analyses from the EMBRACE study. Ann Oncol 21(8 suppl):viii96–viii121
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M et al (2010) Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 28:375–379
Turner N, Tutt A, Ashworth A (2004) Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 4:814–819
Venkitaraman AR (2002) Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171–182
Lambie H, Miremadi A, Pinder SE, Bell JA, Wencyk P, Paish EC et al (2003) Prognostic significance of BRCA1 expression in sporadic breast carcinomas. J Pathol 200:207–213
Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D et al (2007) BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene 26:2126–2132
Tarasewicz E, Jeruss JS (2012) Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle 11:2443–2451
Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN et al (2002) Cyclin E and survival in patients with breast cancer. N Engl J Med 347:1566–1575
Velasco-Velazquez MA, Li Z, Casimiro M, Loro E, Homsi N, Pestell RG (2011) Examining the role of cyclin D1 in breast cancer. Future Oncol 7:753–765
Blagosklonny MV (2013) Aging is not programmed: genetic pseudo-program is a shadow of developmental growth. Cell Cycle 12:3736–3742
Blagosklonny MV (2013) MTOR-driven quasi-programmed aging as a disposable soma theory: blind watchmaker vs. intelligent designer. Cell Cycle 12:1842–1847
Matsuura I, Chiang KN, Lai CY, He D, Wang G, Ramkumar R et al (2010) Pin1 promotes transforming growth factor-beta-induced migration and invasion. J Biol Chem 285:1754–1764
Nakano A, Koinuma D, Miyazawa K, Uchida T, Saitoh M, Kawabata M et al (2009) Pin1 down-regulates transforming growth factor-beta (TGF-beta) signaling by inducing degradation of Smad proteins. J Biol Chem 284:6109–6115
Jeruss JS, Sturgis CD, Rademaker AW, Woodruff TK (2003) Down-regulation of activin, activin receptors, and Smads in high-grade breast cancer. Cancer Res 63:3783–3790
Acknowledgments
We would like to thank Sarah Jackson for her assistance in reviewing the genomic data presented in the manuscript. We would also like to acknowledge the patients who were brave enough to participate in this clinical trial.
Conflict of interest
V Kaklamani and S. Jain are consultants for EISAI. E. Hughes, K. Timms, A. Gutin, V. Abkevich, Z. Sangale, C. Solimeno, K. Brown, J. Jones, and AR Hartman are employees of Myriad Genetics, Inc. and Myriad Genetic Laboratories, Inc. and receive salaries and stock. J. Jeruss, K. Siziopikou, C. Meservey, B. Jovanovic, I. Helenowski, S. Khan, K. Bethke, N. Hansen, R. Uthe, S. Giordano, S. Rosen, K. Hoskins, J. Von Roenn, S. Jain, and V. Parini have no conflicts to disclose.
Financial support
VGK was financially supported by Dolores Knes Fund, Lynn Sage Foundation, and Harris Family Fund, and JSJ was financially supported by Central Surgical Association, Society of Surgical Oncology, Saslow Family and A Sister’s Hope (JSJ) under the grant nos. NIH K22 CA138776 and R01GM097220. This work was specifically funded by the Society of Surgical Oncology/Susan G. Komen of the Cure Clinical Investigator Award (JSJ). This clinical trial was supported by EISAI. Genomic assays were performed at Myriad Genetics, Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaklamani, V.G., Jeruss, J.S., Hughes, E. et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast Cancer Res Treat 151, 629–638 (2015). https://doi.org/10.1007/s10549-015-3435-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3435-y