Abstract
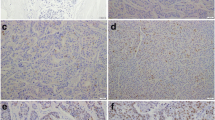
The identification of women with early-stage breast cancer who will develop distant metastasis may improve clinical management. The transcriptional regulator Enhancer of Zeste-2 (EZH2) is overexpressed in invasive breast carcinoma compared with benign breast tissues, with maximal expression in breast cancer metastasis. In this article, our purpose was to investigate the performance of EZH2 protein detection as a predictor of metastasis in women with early-stage breast cancer, which is unknown. We developed a cohort of 480 women with stage I–IIA breast cancer diagnosed between 1996 and 2002 and recorded detailed sociodemographic, clinical, and pathological information. Tumors were histologically characterized and arrayed in tissue microarrays containing 1,443 samples. The nuclear EZH2 expression was investigated by immunohistochemistry and was scored as 1–2 (negative and weak) or 3–4 (moderate and strong) using a validated scoring schema. Scores 1–2 were considered low EZH2; scores 3–4 were considered high EZH2. In this study, we found that after a median follow up of 9 years (range 0.04–14.5 years) 46 of 480 patients (9.6%) developed distant metastasis. High EZH2 was associated with larger size, high histological grade, negative hormone receptors, and first degree family history of breast and/or ovarian carcinoma. While EZH2 could not predict survival in the entire cohort, high EZH2 was a predictor of disease-specific survival in patients with early-stage disease and first degree family history (log rank P value 0.05). Importantly, in this group of patients, high EZH2 was an independent predictor of distant metastasis up to 15 years after primary carcinoma diagnosis (hazard ratio 6.58, 95% CI: 1.40–30.89, P = 0.016) providing survival information above and beyond currently used prognosticators. In conclusion, EZH2 may be a useful biomarker of long-term metastatic risk in women with familial early-stage breast cancer, and warrant further validation studies.

Similar content being viewed by others
References
Sarrazin D, Dewar JA, Arriagada R et al (1986) Conservative management of breast cancer. Br J Surg 73:604–606
Llombart-Cussac A (2008) Improving decision-making in early breast cancer: who to treat and how? Breast Cancer Res Treat 112(Suppl 1):15–24
Laible G, Wolf A, Dorn R et al (1997) Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J 16:3219–3232
Satijn DP, Otte AP (1999) Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta 1447:1–16
Ringrose L, Paro R (2004) Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet 38:413–443
Haupt Y, Alexander WS, Barri G, Klinken SP, Adams JM (1991) Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65:753–763
Hess JL (2004) MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med 10:500–507
Boyer LA, Plath K, Zeitlinger J et al (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441:349–353
Levine SS, Weiss A, Erdjument-Bromage H, Shao Z, Tempst P, Kingston RE (2002) The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol Cell Biol 22:6070–6078
Gonzalez ME, Li X, Toy K et al (2009) Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 28:843–853
Kleer CG, Cao Q, Varambally S et al (2003) EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 100:11606–11611
Cao Q, Yu J, Dhanasekaran SM et al (2008) Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27:7274–7284
Zeidler M, Varambally S, Cao Q et al (2005) The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia 7:1011–1019
Chang CJ, Yang JY, Xia W et al (2011) EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell 19:86–100
Gonzalez ME, DuPrie ML, Krueger H, Merajver SD, Ventura AC, Toy KA, Kleer CG (2011) Histone methyltransferase EZH2 induces Akt-dependent genomic instability and BRCA1 inhibition in breast cancer. Cancer Res 71:2360–2370
Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K (2003) EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 22:5323–5335
Bachmann IM, Halvorsen OJ, Collett K et al (2006) EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 24:268–273
Collett K, Eide GE, Arnes J et al (2006) Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 12:1168–1174
Raaphorst FM, Meijer CJ, Fieret E et al (2003) Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 5:481–488
Varambally S, Dhanasekaran SM, Zhou M et al (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629
Gown AM (2008) Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol 21(Suppl 2):S8–S15
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235
Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG (2006) Identification of EZH2 as a molecular marker for a precancerous state in morphologically normal breast tissues. Cancer Res 66:4095–4099
Puppe J, Drost R, Liu X et al (2009) BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to Polycomb Repressive Complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer 11:R63
Chabner E, Nixon A, Gelman R et al (1998) Family history and treatment outcome in young women after breast-conserving surgery and radiation therapy for early-stage breast cancer. J Clin Oncol 16:2045–2051
Ding L, Kleer CG (2006) Enhancer of Zeste 2 as a marker of preneoplastic progression in the breast. Cancer Res 66:9352–9355
Pietersen AM, Horlings HM, Hauptmann M et al (2008) EZH2 and BMI1 inversely correlate with prognosis and TP53 mutation in breast cancer. Breast Cancer 10:R109
Arisan S, Buyuktuncer ED, Palavan-Unsal N, Caskurlu T, Cakir OO, Ergenekon E (2005) Increased expression of EZH2, a polycomb group protein, in bladder carcinoma. Urol Int 75:252–257
Raman JD, Mongan NP, Tickoo SK, Boorjian SA, Scherr DS, Gudas LJ (2005) Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res 11:8570–8576
Weikert S, Christoph F, Kollermann J et al (2005) Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med 16:349–353
Matsukawa Y, Semba S, Kato H, Ito A, Yanagihara K, Yokozaki H (2006) Expression of the enhancer of zeste homolog 2 is correlated with poor prognosis in human gastric cancer. Cancer Sci 97:484–491
Breuer RH, Snijders PJ, Smit EF et al (2004) Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia 6:736–743
Sudo T, Utsunomiya T, Mimori K et al (2005) Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 92:1754–1758
Acknowledgments
The authors thank Mrs. Robin Kunkel for artwork and Javed Siddiqui for his assistance with tissue microarray construction. This study was financially supported by the National Cancer Institute at the National Institutes of Health (R01 CA125577, UO1 CA154224, and 2R01 CA107469) to CGK; the Department of Defense Breast Cancer Research Program to SHA; the University of Michigan Comprehensive Cancer Center Core Grant (NIH/NCI 5 P30 CA46592); and the Josephine Ford Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alford, S.H., Toy, K., Merajver, S.D. et al. Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat 132, 429–437 (2012). https://doi.org/10.1007/s10549-011-1591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1591-2